Review: Journal of Drug and Alcohol Research (2023) Volume 12, Issue 3
The Effectiveness of using Mefenamic Acid Drug against Primary Dysmenorrhea in Female Adolescents
Ubaid Fuad Frahman1 and Donna Adriani2*2Department of Phisiology, Universitas Trisakti, Indonesia
Donna Adriani, Department of Phisiology, Universitas Trisakti, Indonesia, Email: donna.adriani@trisakti.ac.id
Received: 29-Mar-2023, Manuscript No. JDAR-23-99011 ; Editor assigned: 31-Mar-2023, Pre QC No. JDAR-23-99011 (PQ); Reviewed: 14-Apr-2023, QC No. JDAR-23-99011 ; Revised: 19-Apr-2023, Manuscript No. JDAR-23-99011 (R); Published: 26-Apr-2023, DOI: 10.4303/JDAR/236231
Abstract
Menstrual pain or dysmenorrhea is a gynecological problem in women of all ages, specifically adolescents. Approximately 90% of female adolescents experience menstrual problems, with over 50% of these being dysmenorrhea, which can be divided into primary and secondary types. Furthermore, secondary dysmenorrhea is caused by abnormalities in the genital organs. At the onset of menstruation, there is a decrease in the levels of prostaglandins and estradiol, which leads to the formation of arachidonic acid. Subsequently, this acid is converted into prostaglandins, prostacyclin, thromboxane A2, and Cyclooxygenase (COX). The substances stimulate the work of the uterine muscles, resulting in increased contractions. The pain felt is usually concentrated in the suprapubic area and can be accompanied by other disorders such as digestive problems, nausea, vomiting, constipation, and headaches. This can disrupt daily activities, both from school and work. For dysmenorrhea sufferers, treatment is conducted by visiting the doctor or through self-medication. The most commonly used treatment is Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), with mefenamic acid being one easily accessible NSAID that does not require a prescription and can help reduce menstrual pain. Many factors can cause dysmenorrhea in women, such as genetic, sociodemographics, and pathological conditions. However, further research is needed on the effectiveness of mefenamic acid against primary dysmenorrhea in female adolescents. This literature review aims to determine the effectiveness of using mefenamic acid against primary dysmenorrhea in female adolescents.
Keywords
Dysmenorrhea; NSAIDs; Mefenamic acid; Female adolescents
Introduction
Dysmenorrhea, also known as menstrual pain, is a gynecological problem often found in women of all ages, specifically adolescents between 17 and 25. This is due to high levels of prostaglandins triggering pain during menstruation which can disrupt daily activities, resulting in absence from lessons or work [1,2].
Various factors influence this condition, including psychological factors in women of reproductive age. Moreover, nutritional status, age, history of early menarche, food inintake, and genetics are also reported to cause dysmenorrhea [3]. Complaints of dysmenorrhea vary from mild to severe intensity [4]. Judging from the severity of pain, dysmenorrhea is divided into mild, moderate, and severe categories [3].
The highest number of sufferers is at 17-25 because that age is when they will face various demands due to psychological, physical, and social changes [5]. Dysmenorrhea is the most common menstrual symtomps among adolescents female [6]. The prevalence in Indonesia is recorded at 64.25%, and the literature states that about 87.7% of adolescents in Surakarta experience dysmenorrhea [5].
Pharmacological and non-pharmacological treatments are needed to deal with menstrual pain. One of the treatment options used is to consume mefenamic acid, which is an analgesic easily available, sold freely, and does not require a prescription from a doctor. Furthermore, a systematic review explained that mefenamic acid is selected because it is available and sold freely to relieve dysmenorrhea. Apart from this treatment, non-drug therapy can be adopted, such as compressing the painful part with warm water [7].
In literature research, NSAIDs are very effective in relieving dysmenorrhea. It was stated that 18% of women who were given a placebo experienced moderate to good pain reduction, and a more significant effect was found in 45% and 53% who used NSAIDs [2]. Meanwhile, Ayu MR et al. stated that there was a sufficient relationship between the degree of dysmenorrhea and the use of NSAIDs such as ibuprofen and mefenamic acid [8]. Tabari NM et al. reported that mefenamic acid could reduce complaints of primary dysmenorrhea better than stretching exercises [9]. This contrasts the literature research from Olaodosu FA, where mefenamic acid could be ineffective compared to other NSAIDs [10]. In addition, Xuan Feng stated that using mefenamic acid was ineffective compared to ibuprofen, flurbiprofen, and tamarind peraprofenik in reducing complaints of primary dysmenorrhea [11]. The same result was also stated by Kumar S et al. which researched 68 patients with dysmenorrhea. Even though mefenamic acid controls bleeding and pain in dysmenorrhea, it is not better than diclofenac which is more effective in providing control [12].
Based on the description above, it is interesting to write a literature review regarding the effectiveness of using mefenamic acid against primary dysmenorrhea in female adolescents. This review aims to determine the effectiveness of using mefenamic acid against primary dysmenorrhea in female adolescents. It is expected to provide additional literature and knowledge for medical science and the health sector to improve the health and quality of life of women with primary dysmenorrhea.
Content
Dysmenorrhea
Dysmenorrhea or menstrual pain is a gynecological problem often found in women of various ages, which can cause a decrease in quality of life [2,3]. The term is used to describe menstrual pain that is severe enough to interfere with daily activities, hence, patients are forced to seek assistance through medical intervention or self-medication [2,5].
The prevalence of dysmenorrhea reported in the literature varies, but a greater prevalence is found in females or late adolescents aged 17-25, about 67% to 90%. Furthermore, 90% of female adolescents worldwide experience problems during menstruation, and more than 50% experience primary dysmenorrhea, with 10%-20% being quite severe [3]. Based on 2018 and 2012 data, the prevalence of dysmenorrhea in Indonesia and Surakarta is 60%-70% and 87.7%, respectively [7]. Research in Australia stated that dysmenorrhea was often found in Senior High School students, with a proportion of about 93% but varied between 15% and 75% in adult women. Based on the quality of life, about 41% experienced disturbance, and 7% to 15% rarely felt any disturbance to their quality of life [13].
The clinical results showed that dysmenorrhea is divided into primary and secondary [2,13]. Primary dysmenorrhea is menstrual pain that appears without any abnormalities in the genital organs and occurs after menarche or 12 months due to menstrual cycles [4,13]. The pain occurs before or at the start of menstruation and lasts for several minutes to hours or days [5]. The nature of the pain is cramping in the suprapubic and can spread to the waist and thighs, along with pain in nausea, vomiting, headaches, diarrhea, and irritability [9,13]. Several factors play a role as a cause of primary dysmenorrhea, including age less than 20 years, high socio-economic status, the habit of drinking coffee, smoking, psychiatric factors, constitutional factors, endocrine factors, and allergic factors [14,15]. Meanwhile, secondary dysmenorrhea is menstrual pain with abnormalities in the genital organs. It is a form of menstrual pain due to certain diseases related to the female reproductive organs, and the onset may be years after menarche. This occurs due to various pathological conditions such as endometriosis, salpingitis, myoma, submucosa, corpus uteri polyp, endometritis, retroflection uteri fixate, cervical canal stenosis, and IUD. The pain felt is almost the same as primary dysmenorrhea [9,13].
Based on the pain intensity, dysmenorrhea is divided into mild, moderate, and severe degrees [9,16]. This pain can impact the ability to carry out daily activities. The Multidimensional Scoring of Andersch and Milsom classifies dysmenorrhea pain as follows [16]:
• Mild dysmenorrhea is characterized by menstrual pain without activity restrictions, no need for analgesics, and no systemic complaints.
• Moderate dysmenorrhea is characterized by menstrual pain that affects daily activities, with the need for pain relief analgesics and various systemic complaints.
• Severe dysmenorrhea is characterized by menstrual pain with severe limitations in daily activities, minimal analgesic response to pain relief, and systemic complaints such as vomiting and fainting.
The degree of dysmenorrhea based on the WaLLID Scorecan be seen in Table 1.
| Workability | Location | Pain intensity | Pain duration |
|---|---|---|---|
| 0: Never | 0: None | 0: No pain | 0: 0 day |
| 1: Rarely | 1: 1 place | 1: Slight pain | 1: 1-2 days |
| 2: Almost always | 2: 2-3 places | 2: Slightly more painful | 2: 3-4 days |
| 3: Always | 3: 4 places | 3: Very sick | 1: ≥ 5 days |
Note: 0 point=No dysmenorrhea, 1-4 points=Mild dysmenorrhea, 5-7 points=Moderate dysmenorrhea, 8-12 points=Severe dysmenorrhea
Table 1: WaLLID score.
Genetic and emotional factors play important roles in developing dysmenorrhea, which is severe pain during menstruation. Early onset increased flow during menstruation, and a family history of dysmenorrhea have been linked to the condition [5,17]. This research also showed that stress levels and socio-economic status can affect dysmenorrhea [18,19]. Secondary dysmenorrhea is caused by pathological conditions such as endometriosis or ovarian cysts. Risk factors commonly associated with the condition include early onset of menstruation, family history of dysmenorrhea, abnormal body mass index, fast food consumption, duration of bleeding during menstruation, exposure to cigarette smoke, coffee consumption, and alexithymia [5,20,21].
Primary dysmenorrhea is common in women, who have not given birth or are under the age of 25, and characterized by pain in the suprapubic area 1 day before menstruation and lasting for 2 days, which can radiate to the surrounding areas, such as the buttocks, lower back, and legs. Dysmenorrhea can also accompany other symptoms such as nausea, vomiting, digestive disorders, constipation, dizziness, back pain, mood changes, and headaches. In primary dysmenorrhea, no abnormalities are found in the genital organs [13,14].
Treatment is divided into pharmacological and non-pharmacological methods [9]. Pharmacological treatment involves using drugs similar to prostaglandin inhibitors, such as Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), which inhibit the production and function of prostaglandins. These drugs should not be given to pregnant women with digestive disorders, asthma, or allergies to similar anti- prostaglandin drugs. Other drugs used to treat dysmenorrhea include analgesics and hormonal treatment [9].
Non-pharmacological treatment can be carried out by performing stretching or flexibility exercises. Compressing the lower abdomen with warm water can relax muscles and the nervous system. Furthermore, regular exercise can increase endorphins, which are natural painkillers. Using distraction strategies such as music listening for light to moderate discomfort, massages can diminish pain reactions, and breathing relaxation techniques can reduce the intensity of dysmenorrhea [9,22,23].
Mefenamic acid
Mefenamic acid is a phenamat derivative of anthranilic acid, which acts as a pain reliever and is classified as a Non-Steroidal Anti-Inflammatory Drug (NSAID) [24]. Moreover, it has anti-inflammatory effects, and mefenamic acid also has the function of antipyretic and analgesic that works by inhibiting the synthesis of prostaglandins as an inflammatory mediator. It can treat mild to moderate pain complaints, such as dysmenorrhea, menorrhagia, and toothache. In addition, it can also relieve pain in musculoskeletal disorders, such as osteoarthritis and postoperative pain. In the analgesia test, mefenamic acid is the only phenamat that shows central and peripheral activity. Phenamat compounds also can inhibit Cyclooxygenase (COX) enzymes. Contraindications for mefenamic acid include not being given to patients with a history of gastrointestinal diseases such as chronic gastritis, children under the age of 14, and pregnant women because of the risk of toxicity to the fetus [12,24].
The pharmacokinetics of mefenamic acid involve it being absorbed primarily in the stomach and intestines, then passing through the liver into the blood, and carried by the blood to the processing site. About 90% are bound to plasma proteins, which can be used cautiously in people receiving anticoagulants. The plasma peak level is reached within 2 to 4 hours after the first administration of 2 mg × 500 mg and continued with 3-4 × 500 mg with a half-life of 2 hours for 7 days. About 50% of the dose is excreted in the urine as a 3-hydroxymethyl conjugated metabolite, and 20% is found in feces as a non-conjugated 3-carboxyl metabolite [24,25]. Mefenamic acid can be swapped out for tiaprofenic acid or flurbiprofen when it does not help with the pain in the cycle [11].
The pharmacodynamics of mefenamic acid is to reduce pain and inflammation by inhibiting the COX enzyme to disrupt the conversion of arachidonic acid to prostaglandins. There are 2 isoforms of the COX enzyme, namely COX-1 and COX-2, encoded by different genes, and their expression is unique, sharing 63% identical amino acids and having the same catalytic bond. COX-1 is constitutive, and the presence is always constant without the influence of a stimulus, while COX-2 is an inducible enzyme influenced by a stimulus [24,25].
COX-1 is important in maintaining normal function in various body tissues, particularly the kidney, gastrointestinal tract, and platelets. In the gastric mucosa, this gene produces prostacyclin, which is cytoprotective. Furthermore, COX-2 is induced by various inflammatory stimuli such as cytokines, endotoxins, and growth factors. This enzyme also has physiological functions in the kidney, vascular tissue, and tissue repair processes [9,10].
Thromboxane A2, synthesized by COX-1, causes platelet aggregation, vasoconstriction, and smooth muscle proliferation. In contrast, prostacyclin synthesized by COX-2 in the endothelial cells counteracts these effects and causes platelet inhibition, vasodilation, and anti-proliferative effects [26,27]. Mefenamic acid has a stronger ability to inhibit COX-1 compared to COX-2. Therefore, mefenamic acid has a stronger pain-relieving effect than an anti-inflammatory effect [24].
Since every drug has side effects, mefenamic acid, one of the NSAIDs, is recommended not to be consumed for longer periods of more than 7 days. It can cause gastrointestinal problems such as stomach irritation, dyspepsia, hypersensitivity, and diarrhea, as well as in patients with a low immunity status with HIV/AIDS may experience bloody diarrhoea [24].
Treatment for primary dysmenorrhea, besides mefenamic acid, can also use herbs, physical intervention therapy such as stretching therapy, and other NSAIDs. Various NSAIDs can be used as analgesics and anti-inflammatory agents through COX enzyme inhibitors (COX-1 and COX-2). Other types of NSAIDs include flurbiprofen, ketoprofen, naproxen, tiaprofenic, ibuprofen, and aspirin [11].
Research showed ginger and turmeric can be used as herbal alternatives to reduce dysmenorrhea symptoms. The use of ginger or turmeric extracts at a dose of 250 mg every 6 hours is reported to have the same pain-reducing effect as mefenamic acid and does not have gastrointestinal side effects. Herbs have a significant result during the first month of the menstrual cycle (p=0.01), while when consumed in the following months, there is a decrease in the efficiency of the extract in addressing pain symptoms (p=0.04). Therefore, it is recommended to use a combination of mefenamic acid and turmeric or ginger extract to achieve more significant pain relief. Ginger and turmeric relieve dysmenorrhea by inhibiting prostaglandin and thromboxane [26,27].
Studies comparing different NSAIDs found that flurbiprofen, paparofenic, and mefenamic acid are the most effective treatments for relieving primary dysmenorrhea symptoms. Eachrofenic and mefenamic acids are the safest types of NSAIDs because there are very few reports of side effects after taking the drug. Furthermore, flurbiprofen needs to be researched to determine long-term side effects. Refocoxib and valdecoxib can cause cardiovascular issues, whereas indomethacin minimizes complaints of dysmenorrhea but is associated with the most commonly reported side effect, gastrointestinal complaints. Aspirin is not recommended because the research stated it was inefficient in reducing complaints of primary dysmenorrhea [11].
Effectiveness of using mefenamic acid with dysmenorrhea
The early menstrual phase is characterized by a simultaneous decrease in circulating prostaglandins and estradiol. This allows the initiation of pro-inflammatory cytokines, Matrix Metalloproteinases [MMPs], and transcription of endometrial collagen, as shown in Figure 1. The decline of progesterone and estrogen levels at the end of the luteal phase triggers a cascade leading to damage of the endometrial tissue, release of phospholipid layers, and subsequent prostaglandin production.
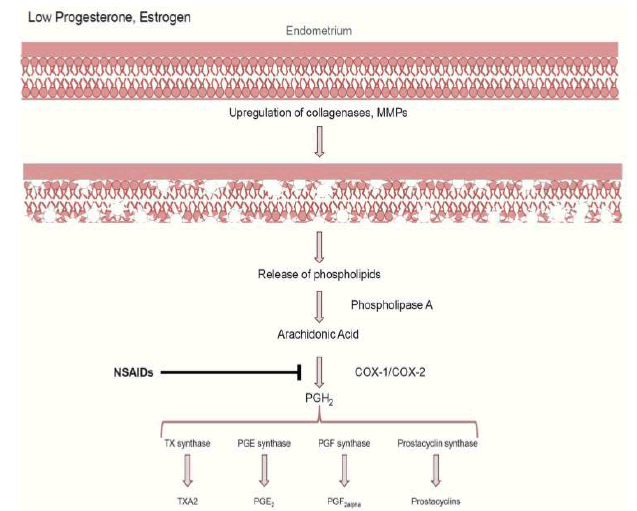
Figure 1: Production of prostagladins in dysmenorrhea.
COX: Cyclooxygenase, MMPs: Matrix Metalloproteinases, NSAIDs: Non-Steroid Anti Inflammation Drugs, PG: Prostaglandin, TX: Tromboksan
The resulting impact is that the MMPs liberate the phospholipid layer and cellular membranes, breaking down the endometrial tissue [10,28].
This can increase the enzyme phospholipase A2 to form arachidonic acid. Furthermore, arachidonic acid will be synthesized into prostaglandins, prostacyclin, thromboxane A2, as well as COX-1 and COX-2. The substances formed can stimulate uterine muscle work resulting in increased contractions [2,29]. Based on the literature, the expression of COX 2 is highest during menstruation but does not play a role in dysmenorrhea [10,29].
Literature suggested that the final production of PGE2 and PGF 2α is found to increase in cases of dysmenorrhea. The identification of increased PGE2 and PGF 2α supports the strategy of inhibiting COX-1 and COX-2 using non-steroidal anti-inflammatory drugs [OAINS] to manage pain [29,30]. Mefenamic acid can bind to COX-1 and COX-2, inhibiting prostaglandins’ synthesis and lowering the pain threshold [24].
Discussion
Pain during menstruation, known as dysmenorrhea, is common among teenagers and can affect a woman’s quality of life. Mefenamic acid is a type of fenamate drug that belongs to Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and is related to dysmenorrhea due to its ability to reduce pain intensity. This statement is supported by the research of Ningsih R et al. (2013), using a quasi-experimental design on 64 female adolescents. It showed mefenamic acid significantly reduced pain intensity in female adolescents with dysmenorrhea (p=0.002) [31]. This is consistent with the findings of Gomathy N et al., where using mefenamic acid is effective and remains a choice in treating primary dysmenorrhea (p=0.001). Even though it improves symptoms, its benefits are not long-lasting as complaints improve only in the cycle, where patients can still experience recurrent pain. Therefore, mefenamic acid is consumed in the following cycles to achieve the same effect of reducing pain intensity during menstruation [8].
The research by Alices Y et al. in Padang, West Sumatra, on 70 respondents with a cross-sectional design found that using mefenamic acid can reduce menstrual pain with a statistical test of the Spearman rank correlation. The result obtained r=0.000 (r<0.05), which means there is a correlation between the two variables [8]. The results are also supported by Abadian K et al. which conducted research on 70 respondents and found a significant pain reduction (p=0.001) in women who consumed mefenamic acid compared to those who consumed the placebo [33]. It has been identified that in cases of dysmenorrhea, there is an increase in the production of PGE2 and PGF 2α, causing pain. Mefenamic acid, which can act as a COX inhibitor, is utilized to manage pain caused by its ability to inhibit the binding of PGE2 to its receptor. Therefore, converting arachidonic acid into prostaglandin is disturbed, and it can lower the pain threshold [24].
Sugumar R et al. stated that NSAIDs effectively reduce dysmenorrhea complaints with mild to moderate intensity. NSAIDs effects such as reducing the amount of menstrual blood and alleviating other symptoms such as headaches, bloating, diarrhea, and breast pain. Therefore, the use of mefenamic acid is effective in treating primary dysmenorrhea because it suppresses the production of prostaglandins in the endometrium, reducing cramps and restoring normal uterine activity [34]. In the research by Masoumi SZ et al. on 127 women with primary dysmenorrhea at Hamadan University from February 2012 to April 2013, the intensity and duration of pain were significantly reduced after consuming mefenamic acid during the first 3 days of menstruation (p=0.001). In addition to reducing the degree of dysmenorrhea discomfort by suppressing prostaglandin synthesis, mefenamic acid also reduces the duration and quantity of menstrual flow, as well as other symptoms generated, such as headaches, bloating, and diarrhoea [35].
In the research conducted by Farahani ELA et al. from May 2013 to March 2014 on women with dysmenorrhea in the area around Arak University, Iran, the group was given 250 mg of mefenamic acid capsules with the first dose of 2 capsules followed by 1 every 6 hours for 2 days, and the result was a decrease in perceived pain severity (p<0.001) [36]. Hesami S et al. explained that using ibuprofen 400 mg and 250 mg of mefenamic acid and the herbal ginger plant did not significantly reduce pain intensity. The extract only reduced complaints of dysmenorrhea pain in the first cycle and did not show a significant effect. In contrast to giving mefenamic acid 500 mg twice daily, it significantly reduced primary dysmenorrhea pain in the first and subsequent monthly cycles. Giving mefenamic acid 250 mg in combination with turmeric herbs is an alternative for the following monthly cycle to reduce pain in primary dysmenorrhea. Furthermore, the COX pathway in arachidonic acid metabolism is the most active in utero. Estrogen increases prostaglandins when menstruation reaches its peak. Progesterone inhibits prostaglandin synthesis until menstruation begins. Higher concentrations of prostaglandins were found in women with menstrual pain compared to those without menstrual pain. In the endometrium, PGE2 acts as a potent platelet dispersing agent and vasodilator, while PGF2α is a mediator and trigger of pain sensation [27,29]. Based on these findings, suppression of prostaglandins is an effective way to reduce pain during menstruation. Inhibition of NSAIDs prostaglandin synthesis is a mechanism. Mefenamic acid, an effective COX inhibitor from the NSAID class, is one of the alternatives in pain relief therapy for women with primary dysmenorrhea.
The research conducted by Tabari NM comparing the effectiveness of reducing pain with stretching exercises and using mefenamic acid for 3 months found that the compound gave better results in reducing pain than stretching exercises for treating primary dysmenorrhea in the short term but obtained better results in stretching exercises in the long-term [9]. The literature from Oladosu et al. stated that NSAIDs such as mefenamic acid might be less effective than naproxen, diclofenac, ketorolac, or other propionic acid derivatives. This is caused because there are influential factors such as variations in the response and tolerance of individual patients in reducing complaints of pain during menstruation. However, not a few women experienced resistance to NSAIDs. This resistance can occur for reasons that are not clear but related to pathological mechanisms contributing to treatment unresponsiveness. Several causes include anatomic abnormalities resulting in secondary dysmenorrhea, drug absorption, gene susceptibility, COX, leukotriene, platelets, prostaglandin-mediated pathways, and other molecular mechanisms suspected as the cause of resistance. Women with menstrual pain can experience resistance to a drug from the NSAID group when they have received treatment with the same type for 3 cycles, but there is no improvement [10].
Conclusion and Suggestions
Dysmenorrhea is a prevalent issue among adolescent females, with about 90% experiencing pain during menstruation, and over half of these cases is primary dysmenorrhea. One solution is the use of NSAIDs, specifically mefenamic acid, which has been shown to effectively reduce pain in primary dysmenorrhea, allowing sufferers to maintain their daily activities and quality of life. Further research on the effects of other analgesics is needed to compare their effectiveness and determine factors that influence the effectiveness of mefenamic acid.
Acknowledgement
None.
Conflict of Interest
Authors have no conflict of interest to declare.
References
- W.P. Sari, D.H. Harahap, M.I. Saleh, Prevalence of the use of anti-inflammacinon-steroid (NSAIDS) dismenored relieves faculty of medical Sriwijayapalembang University, Majalah Kedokteran Sriwijaya, 50(2018):154-68.
- R. Ningsih, Seyowati, H. Rahmah, Effectiveness of pain relief packages in adolescents with dysmenorrhea, Jurnal Keperawatan Indonesia, 16(2013):67–76.
- T.A. Larasati, F. Alatas, Primary dysmenorrhea and risk factors for primary dysmenorrhea in adolescents, Jurnal Majority, 5(2016):79-83.
- V.G. Carolina, A. Devita, Correlation of calcium intake and the intensity of primary dysmenorrhea in adolescents, J Biomedika Kesehat, 5(2022).
- D. Wrisnijati, B. Wiboworini, Sugiarto, Prevalence and factors related to the degree of dysmenorrhea in young women in Surakarta, 3(2019):76-89.
- W. Kuncoroaji, I. Rachmiyani, Omega-3 fatty acids relieve dysmenorrhea female senior high school students, Int J Pharm Res, 12(2020):2633-40.
- J. Marjoribanks, R.O. Ayeleke, C. Farquhar, M. Proctor, Nonsteroidal anti-inflammatory drugs for dysmenorrhoea, Cochrane Database Syst Rev, 7(2015):130-79.
- M.R. Ayu, Y. Alices, Rahmatini, The relationship between the degree of dysmenorrhea pain and the use of non-steroidal anti-inflammatory drugs, Jurnal FK Andalas, 4(2015):551-55.
- N.M. Tabari, M.A. Shirvani, A. Alipour, Comparison of the effect of stretching exercises and mefenamic acid on reduction of pain and menstruation characteristics in primary dysminore: A randomized clinical trial, Oman Med J, 32(2017):47-53.
- F.A. Oladosu, F.F. Tu, K.M. Hellman, NSAID resistance in dysmenorrhea: Epidemiology, causes, and treatment, Am J Obstet Gynecol, 218(2018):390-400.
- X. Feng, X. Wang, Comparison of the efficacy and safety of non-steroidal anti-inflammatory drugs for patients with primary dysmenorrhea: A network meta-analysis, Mol Pain, 14(2018):1-14.
- S. Kumar, U. Tekur, B. Singh, D. Kumar, Mefenamic acid and diclofenac in the treatment of menorrhagia and dysmenorrhea in dysfunctional uterine bleeding: A randomized comparative study, Int J Basic Clin Pharmacol, 7(2018):1905-11.
- H. Ju, M. Jones, G. Mishra, The prevalence and risk factors of dysmenorrhea, Epidemiol Rev, 232(2014): 104-13.
- S. Shah, K. Makwana, P. Shah, Menstrual characteristic and prevalence of dysmenorrhea among female physiotherapy students, Int J Med Res Health Sci Res, 1(2015):1-8.
- Handayani, L.I. Gamayanti, M. Julia, Dysmenorrhea and anxiety in adolescents, Sari Pediatri, 15(2013):27-31.
- D. Moghadam, A. Khosravi, Verbal multidimensional scoring system with visual analogue scale for evaluating of shirazi Thymus vulgaris on menstrual pain, J Pharm Biomed, 23(2012):1-5.
- H. Hutomo, Factors influencing the degree of dysmenorrhea pain reduction in PKK members, Jurnal Universitas Sumatera Utara, (2014):1-13.
- S. Sevil, O. Kevser, U. Alaatin, R. Arslan, Review of frequency of dysmenorrhea and some associated factors and evaluation of the relationship between dysmenorrhea and sleep quality in university students, Gynecol Obstet Invest, 78(2014):179-82.
- D. Sari, A.E. Nurdin, Defrin, Correlation between stress and primary dysmenorrhea in medical students, Faculty of Medicine, Andalas University, Jurnal Kesehatan Andalas, 4(2015): 567-70.
- S. Charu, R. Amita, R. Sujoy, G.A. Thomas, Menstrual characteristic and prevalence and effect of dysmenorrhea on quality of life of medical students, IJCRIMPH, 4(2012):276-94.
- Danielle, O.F. Balog, Early female puberty: A review of research on etiology and implication, Health Educator, 41(2009):47-53.
- A.S. Osayande, S. Mehulic, Diagnosis and initial management of dysmenorrhea, Am Fam M Physician, 89(2014):341-6.
- A. Rakhma, Gambaran derajat nyeri dismenore dan upaya penanganannya pada siswi sekolah menengah kejuruan arjuna depok jawa barat, Jurnal UIN Syarif Hidayatullah, (2012):16-20.
- A. Jaish, S. Juma, Mecca, A.M. Thawabteh, Mefenamic acid prodrugs and codrugs: Two decades of development, WJPPS, 4(2015):2408-29.
- E.D. Frust, R.W. Ulrich, S.Prakash, NSAID in Katzung, Betram G: Basic & clinical pharmacology, New Yorik: Lange Medical Book McGraw Hill, 13(2015):715-24.
- M.A. Shirvani, N.M. Tabari, A. Alipour, The effect of mefenamic acid and ginger on pain relief in primary dysmenorrhea: A randomized clinical trial, Arch Gynecol Obstet, 291(2015):1277-81.
- S. Hesami, M. Kavianpour, M.R. Nooshabadi, M. Yousefi, F. Lalooha, et al. Randomized, double-blind, placebo-controlled clinical trial studying the effects of Turmeric in combination with mefenamic acid in patients with primary dysmenorrhoea, J Gynecol Obstet Hum Reprod, 50(2021):101840.
- A. Al-Saeed, Gastrointestinal and cardiovascular risk of non-steroidal anti-inflammatory drugs, Oman Med J, 26(2011):385-391
- S.G. Marx, M.J. Wentz, L.B. MacKay, Effects of progesterone on iNOS, COX-2, and collagen expression in the cervix, J Histochem Cytochem, 21(2006):623-39.
- K.M. Hellman, P.Y.Yu, F.A. Oladosu, C. Segel, A. Han, et al. The effect of platelet activating factor on uterine concractility, perfusion, hypoxia, and pain in mice, Reprod Sci, 25(2018):384-394.
- R. Ningsih, Seyowati, H. Rahmah, Effectiveness of pain relief packages in adolescents with dysmenorrhea, Jurnal Keperawatan Indonesia, 16(2013):67–76.
- N. Gomathy, K.R. Dhanasekar, R. Amirtha, Supportive therapy for dysmenorrhea: Time to look beyond mefenamic acid in primary care, J Family Med Prim Care, 8(2019):3487-3491.
- K. Abadian, Z. Keshavarz, F. Mojab, H.A. Majd, N.M. Abbasi, Comparison the effect of mefenamic acid and Teucrium polium on the severity and systemic symptoms of dysmenorrhea, Complement Ther Clin Pract, 22(2016):12-5.
- R. Sugumar, V. Krishnaiah, G.S. Channaveera, Mruthyunjaya, Comparison of the pattern, efficacy, and tolerability of self-medicated drugs in primary dysmenorrhea: A questionnaire based survey, Indian J Pharmacol, 45(2013):180-3.
- S.Z. Masoumi, H.R. Asl, S.R. Oliaei, J. Poorolajal, M.H. Panah, Evaluation of mint efficacy regarding dysmenorrhea in comparison with mefenamic acid: Double-blinded randomized crossover study, Iran J Nurs Midwifery Res, 21(2016):363-7.
- E.L. Farahani, S.B.H. Azghdy, H. Kasraei, T. Heidari, Comparison of the effect of honey and mefenamic acid on the severity of pain in women with primary dysmenorrhea, Arch Gynecol Obstet, 296(2017):277–83.
Copyright: © 2023 Ubaid Fuad Frahman, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

