Research Article: Journal of Drug and Alcohol Research (2025) Volume 14, Issue 4
Novel Hypotheses on Neurophatic Pain in Isaacs Sydrome and Drug Therapy
Lourdes de Fatima Ibanez Valdes1 and Humberto Foyaca Sibat2*2Department of Neurology, Nelson Mandela Academic Hospital, Walter Sisulu University, South Africa
Humberto Foyaca Sibat, Department of Neurology, Nelson Mandela Academic Hospital, Walter Sisulu University, South Africa, Email: humbertofoyacasibat@gmail.com
Received: 06-May-2025, Manuscript No. JDAR-25-165177; Editor assigned: 08-May-2025, Pre QC No. JDAR-25-165177 (PQ); Reviewed: 22-May-2025, QC No. JDAR-25-165177; Revised: 12-Aug-2025, Manuscript No. JDAR-25-165177 (R); Published: 19-Aug-2025, DOI: 10.4303/JDAR/236442
Abstract
Introduction: In 2001, we reported two patients with Isaacs' syndrome who responded remarkably well clinically and electromyographically to low oral doses of phenytoin. Isaacs Syndrome (IS) is characterized by recurrent spontaneous muscle activity of the peripheral nerve source that can be triggered by induced muscle contraction or voluntary muscular activity. Some authors have proposed an autoimmune aetiology based on clinical events suggesting a possible autoimmune aetiology, including the presence of oligoclonal bands in the cerebrospinal fluid and a good response to plasmapheresis. The primary aim of this study is to answer the following research questions: What is the pathophysiology of neuritic pain in patients presenting to IS? What is the best drug therapy choice?
Methods: We searched the literature, following the guidelines outlined in the PRISMA statement. From January 2023 to January 2025, the authors searched the scientific databases, Scopus, Embassy, Medline, and PubMed Central using the following searches: "Isaac syndrome" OR "neuropathic pain" OR "therapy of neuropathic pain" OR "treatment of Issac syndrome" OR "therapy of NP and IS", OR "mTOR in NP" OR "mTOR in IS".
Results: After screening the full-text articles for relevance, xx were included for final review. However, no article was selected when we searched for association/NP/IS/Drug therapy.
Conclusions: Based on the information reported by other investigators, we have developed some hypotheses regarding the pathogenesis of this condition and novel drug therapy.
Keywords
Isaac syndrome; Neuropathic pain; mTOR; Therapy of neuropathic pain; Drug therapy of Isaac syndrome
Introduction
In 1961, Isaacs H [1] described a syndrome of continuous muscle-fibre activity, later known as Isaacs syndrome.
In 2001, we reported two patients with Isaacs' syndrome who responded remarkably well clinically and electromyographically to low oral doses of phenytoin. Both cases presented associated neurocysticercosis and hypothesised about peripheral nerve membrane disorders, neuroimmunology mechanisms, and the response to phenytoin. To the best of our knowledge, there have been no reports of good responses to low oral dosages of phenytoin [2].
Isaacs Syndrome (IS) is characterised by recurrent spontaneous muscle activity of the peripheral nerve source that can be triggered by induced muscle contraction or voluntary muscular activity. This uncommon disorder, described in 1961, is characterised by hyperexcitability of peripheral motor nerves, resulting in continuous muscle f ibre activity leading to incapacitating muscle twitching, cramps, and weakness. However, IS (also known as acquired neuromyotonia) may sometimes accompany hereditary neuropathies or other diseases. Several physicians have long recognised IS; however, its rarity, the variability of its clinical manifestation, and ways of presentation are probably the most crucial reasons why it's frequently misdiagnosed or wrongly treated [2].
Some authors have proposed an autoimmune aetiology based on clinical events suggesting a possible autoimmune aetiology, including the presence of oligoclonal bands in the cerebrospinal fluid and a good response to plasmapheresis. They reported 40 patients from the past thirty years, including those with thymoma associated with myasthenia gravis, who raised anti-acetylcholine receptor antibody titters, as well as antibodies against potassium channels of peripheral nerves, among other immunological disorders [2]. At that time, we reported that phenytoin and carbamazepine were efficacious because they modulate neuronal sodium channels, thereby decreasing cellular excitability and nerve impulse axonal propagation. We hypothesised that phenytoin helped differentiate IS from other clinical conditions mimicking pseudo/true myotonia, such as myotonic syndrome, stiff-man syndrome, pyramidal syndromes, stiff limb syndrome, focal lesions of the spinal cord, extrapyramidal syndromes, progressive encephalomyelitis with rigidity, and whether such distinction has implications for aetiology, drug therapy, and outcome [2]. Other authors have considered Isaacs' syndrome, also known as peripheral nerve hyperexcitability or neuromyotonia. This rare autoimmune neurological disorder affects the peripheral nervous system, whose clinical features include fasciculations, muscle cramps, neuromyotonia, excessive sweating, generalized body aches, chronic pain, muscle atrophy, bulbar dysfunction, muscle stiffness, and myokymia due to spontaneous and/ or continuous muscle fibre activity that results from peripheral nerve hyperexcitability and trismus following the contraction of the masseter requiring dental treatment plus plasmapheresis therapy [3,4].
Although the incidence and prevalence has remained unknown, Orphanet reported up to two hundred cases across forty-one countries in 2013 [5].
Patients should be treated symptomatically initially. However, several investigators suggest performing plasmapheresis and reporting outstanding results [3,6,7].
Neuromyotonia is characterized by an initial phase of progressive muscle stiffness (in cases presenting IS), eventually leading to remarkable fasciculations across various muscles. The progression of this clinical manifestation might serve as a hallmark of the disorder and highlight the underlying neuromuscular hyperexcitability [8].
Last year, Sarraf et al. reported twelve cases presenting Isaacs' syndrome, including their clinical features, laboratory parameters, malignancy work-up, electrophysiological f indings, and therapeutic management. They found f ive positive patients for Contactin-Associated Protein like 2 (CASPR2) antibodies and Leucine-Rich Glioma Inactivated 1 (LGI1) antibody. The other five cases were LGI1-negative and CASPR2-positive, and one patient had a negative LGI1 antibody and borderline-positive titters for CASPR2 [9].
In cases under suspicion of IS based on their clinical features. Still, the presence of hallucinations, agitation, confusion, and Insomnia (encephalopathy) alongside neuromyotonia and hyperhidrosis, then the diagnosis of Morvan's syndrome in the PNH group disorders must be considered [10].
The leading cause of Isaacs' syndrome pathophysiology is the dysfunction of Voltage-Gated Potassium Channels (VGKCs), complex proteins that could be associated with an acquired or genetic factor [11].
The acquired presentation is related to antibodies against the VGKC complex, and Leucine-rich Glioma Inactivated 1 (LGI1) and Contactin-Associated Protein-like 2 (CASPR2) have been documented as the two main targets of the VGKC complex [12].
Other authors have reported that Isaacs' syndrome can be present in some non-hematologic and hematologic malignancies, such as thymoma and small cell lung cancer, paraneoplastic syndrome, as well as some autoimmune disorders such as Hashimoto's thyroiditis, pernicious anaemia, myasthenia gravis, rheumatoid arthritis, and celiac disease [13].
Other investigators have reported that KCNA6, KCNQ2, KCNA1, KCNA2, and potassium channel gene mutations are inherited forms of IS [14].
Nerve Conduction Studies (NCS) usually follow normal parameters, except for late response and after discharges on motor NCS. On the other hand, however, Electromyography (EMG) may reveal several spontaneous discharges, including fibrillation potentials, myokymic discharges, fasciculation, and neuromyotonic discharges [15].
The management of IS usually refers to therapy of the underlying autoimmune disorder or associated malignancy if detected, immunomodulation therapy with Intravenous Immunoglobulin (IVIG), symptomatic treatment, and plasma exchange. In some patients, maintenance therapy by administering immunosuppressive therapeutic drugs is strongly suggested [8].
The primary aim of this study is to answer the following research questions: What is the pathophysiology of neuritic pain in patients presenting to IS? What is the best drug therapy choice?.
Materials and Methods
Search strategy
We used the PRISMA guidelines to search the medical literature systematically. From January 2000 to January 2025, the authors searched the scientific databases, Scopus, Embassy, Medline, and PubMed Central using the following searches: "Isaac syndrome" OR "neuropathic pain" OR "therapy of neuropathic pain" OR "treatment of Issac syndrome" OR "therapy of NP and IS", OR "mTOR in NP" OR "mTOR in IS". After removing duplicates, two reviewers (LDFIV and HFS) from each side screened titles and abstracts and evaluated the full texts of eligible articles based on the proposed inclusion criteria. Any disagreement between the reviewers involved in the literature search was resolved through discussion with all authors to reach a consensus.
Selection criteria
The following manuscripts were included in the systematic review:
Articles on Isaac syndrome, neuropathic pain, and Schwann cell disorder in ISA/NP, with detailed pathogenesis and/or drug therapy.
Exclusion criteria were as follows:
• Inaccessibility to full text.
• Articles with unclear pathogenesis/drugs therapy of IS/NP.
• Lack of relevant clinicopathological data.
• Non-original studies (i.e., editorials, letters, conference proceeding, book chapters).
• Animal model studies.
• Non-/Spanish/Portuguese/English studies.
The papers were thoroughly assessed, and duplicates were looked for.
Data extraction and quality assessment
All selected data were processed in an Excel electronic database. That information included pathogenesis, drug management, initial clinical presentation, evaluation of NP/IS after treatment, follow-up, and status at the latest evaluation. The quality of the studies included was categorized as poor, fair or reasonable, in agreement with the criteria of the National Institutes of Health (https://www.nhlbi.nih.gov/health-topics/study-quality assessment-tools, (accessed on March 10, 2025). Two reviewers (LDFIV and HFS) independently evaluated the articles, and the discussion resolved disagreements.
Statistical analysis
The primary objective of this study was to evaluate whether the pathogenesis/drug management of NP/IS differs significantly among different ways of therapies. Without a comprehensive reference for the total number of NP/IS cases, the prevalence of NP/IS responding to drug therapy with associated NP/IS was searched through a comprehensive review of the medical literature.
Statistical analyses were performed using XLSTAT (add on for Microsoft Excel, version 2021.4.1, Addinsoft SARL) and RStudio (version 4.3.1, https://www.rstudio. com/). Variations in continuous variables were assessed using the Mann-Whitney U-test. Fisher's exact test or the Chi-square test was used to evaluate the association among categorical variables, as appropriate. We presented descriptive statistics for continuous variables as median (95% confidence Interval (95% CI)). All situations were evaluated using the Kaplan-Meier method to identify relevant prognosticators. A model of multivariable Cox proportional hazards with a priori selection of covariates was used to check for independent prognostic effects.
To assess the therapy's protective effect, we built a multivariable model that included only OMAS cases. The results are expressed as a p-value and a hazard ratio with a 95% confidence interval. Statistical significance was defined at 0.05.
Results and Discussion
A total of 988 titles were collected from the literature search. After removing duplicates and excluding 349 records, 639 relevant articles were examined. 82 studies were unavailable for retrieving. After including six additional articles identified from citation searching (563), 205 were excluded for several reasons. A total of 358 records were identified from these searches. After exporting the resultant study titles to Excel, duplicate publications that did not meet the inclusion/exclusion criteria were eliminated, leaving 77 distinct titles. We checked the eligibility of all the remaining original full-text articles, abstracts, and titles. Generally, titles were not included if they were unavailable in Spanish, Portuguese, or English or irrelevant to NP/IS. Articles relevant to NP/IS included the clinical presentation, management, treatment, pathogenesis, and treatment. A total of 43 articles remained following this initial screening. These articles were then filtered by the authors using their abstracts. Abstracts that were out-of-date, unrelated to the subject, or not available in Spanish, Portuguese, or English were eliminated after additional review. Six articles remained for full-text review after 39 abstracts were eliminated. Two were selected for final review after the full-text articles were screened for relevancy. At the end no article was selected when we searched for association/NP/ IS/Drug Therapy (DT).
Series description and differences among groups
All the selected studies were relevant to the subject of this systematic review. None were randomized controlled trials or prospective studies; all the articles included were case reports and case series.
The total number of patients presenting NP/IS/Good response to DT was cero.
Median age was 17.5 (range 11-79) with significant differences between age groups (p<0.001). We did not find remarkable variations in gender (p=0.064), although females presenting OMAS were noticeably more frequent and slightly more prevalent.
Comments and final remarks
Brief comments on the role of Schwann cells IS.
By influencing the plasticity and survival of lower motor neurone cells, regulating inflammatory responses, and changing myelination processes, Schwann cells (Sc) are known to be essential for the neurophysiological operation of the Peripheral Nervous System (PNS) based on the work of other researchers [16]. Therefore, potential management planning strategies targeting Sc may support slow disease progression and nerve repair.
The PNS has many glial cells, among which Schwann cells are the best known [17].
Sc is classified as non-myelinating Sc, myelinating Sc, or satellite glia. Sc is categorised as terminal Sc or Remak Sc.
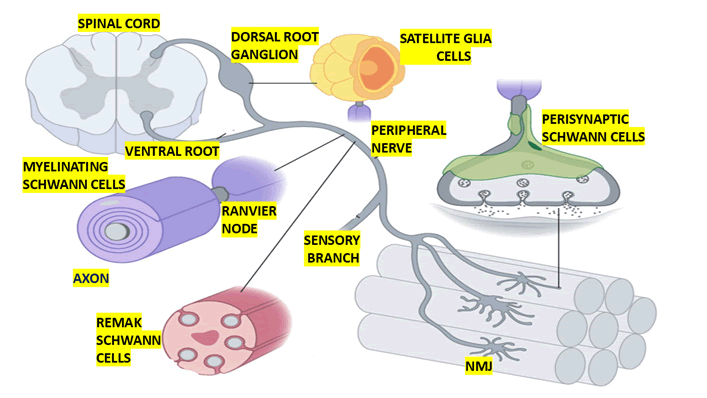
Myelinating Sc spirals around >1 μm peripheral nerve axons to form a compact multilamellar myelin sheath that covers one segment of a single axon and allows saltatory conduction of action potentials (Figure 1).
Figure 1: Show graphical representation of multiple types of Schwann cells in the peripheral nervous system. The location and morphology of Schwann cells, including myelinating Schwann cells, remak Schwann cells, perisynaptic Schwann cells, and satellite glia are depicted. Remak Schwann cells engulf small calibre (<1 μm) peripheral nerve axons to pro vide them with trophic and metabolic support
Schwann cell myelination occurs primarily in A and B fibres, which include medium autonomic neuron axons, motor neuron axons, and some large sensory neuron axons [18].
The embryological development of myelinating Sc is as follows: From neural crest to Sc precursor to immature Sc to pro-myelinating Sc to myelinating Sc [19].
Hundreds of genes, such as Growth Associated Protein 43 (GAP43), Myelin Protein Zero (MPZ), Proteolipid Protein (PLP), Peripheral Myelin Protein 22 (PMP22), and many more, are upregulated during the transition from neural crest to Sc precursor. Precursors of Sc cells follow developing peripheral nerve axons, relying on signals from these developing axons to survive (mostly Neuregulin 1 type III) (NRG1-III) [19].
Precursors for Schwann cells are extremely multipotent and capable of differentiating into a variety of cell types [19]. Once more, gene expression alterations (such as the overexpression of S100, the downregulation of Transcription Factor AP2 (TFAP2A), and the Glial Fibrillary Acidic Protein (GFAP)) are involved in the differentiation of Sc cell precursors into immature Sc cells [20,21].
In addition to developing a thin layer of extracellular matrix (basal lamina), immature Sc has elongated morphologies, greater proliferation, decreased migration, and does not require axonal impulses for survival [19].
Radial sorting, which groups axons based on their calibres to start the process of producing myelinating and Remak Sc, depends on immature Sc. Immature Sc cell groups, which consist of three to eight cells, initiate this process by modulating a common basal lamina and bundling many axons [22]. However, we believe that the process is affected in patients presenting to IS.
However, Immature Sc selects and separates larger calibre axons to the bundle's outer edge by extending lamellipodia-like processes amid the bundled axons. Until medium to large calibre axons establish a 1:1 association with a promyelinating Sc and multiply, immature Sc keeps dividing the axon bundle. Promyelinating Sc creates myelinating Sc and delineates their basal lamina, also known as defasciculation. Nevertheless, Remak Sc will swallow the remaining tiny axons (less than 1 μm in diameter) in the bundle to create Remak bundles once radial sorting is complete. Myelination and radial sorting, as well as, predictably, significant alterations in gene expression, depend on this intricate morphogenetic system and intricate signalling pathways govern these processes [22-25]. We hypothesised that the mechanism is damaged in IS.
We also postulated that the prognosis and recurrent episodes observed in IS may be explained by the failure of the interactions between Sc and axons and the interactivity between Sc and the basal lamina, which together activate signalling cascades and upregulate promyelinating transcription factors like Sox10 and Krox20.
We also thought that the mechanism of sustained hyperexcitability of the peripheral nerve is explained by the failure of the key regulator of myelin sheath thickness (Axonal NRG1-III), as suggested by other authors [26], and mechanical forces acting on the HIPPO pathway, which regulates myelin sheath internodal length, as suggested by others [27,28].
We also speculated that the compaction and spiralling of myelinated Sc cells via the coordination of several cellular processes, such as proper stoichiometric insertion of compact myelin proteins, plasma membrane expansion, lipids, and F-actin assembly and disassembly documented by Salzer [24,29], does not continue in cases of IS.
As a result, the myelin sheaths that are produced do not exhibit the anticipated radial and longitudinal (axial) polarity. As is well known, these sheaths are not continuous, compact, multilamellar structures along the internode's length; rather, they contain small domains, such as nodes, paranodes, and juxtaparanodes at the node of Ranvier, as well as Schmidt-Lanterman incisure transport channels that are irregularly spaced [24]. We postulated that in IS instances, all of these sheaths sustain damage. These separate domains are also involved in this idea at the myelin sheath's exterior or outermost wrap (abaxonal membrane), interior or innermost wrap (axonal membrane, inner mesaxon, and inner collars), outer mesaxon, Cajal bands, appositions, outer collars, and the nucleus) [24] which are also functionally damaged in IS.
We have finally concluded that the mechanism that coordinates the intricate architecture of peripheral nerve myelin is still being developed, with limitations in cases of IS, and that the list of resident proteins localised to the various myelin domains is still expanding, albeit with notable limitations.
Furthermore, since mutations affecting myelinating Schwann cells (Figure 1) produce many forms of hereditary neuropathy, nature has provided some of the most important insights into these cells, including IS, as we hypothesized based on reports made by other authors under different circumstances (injury) [30].
Brief comments on non-myelinating remak Schwann cells
Axons from sensory and autonomic neurones are among the C fibres found in Remak bundles [16]. According to some writers, Remak Sc develops from the same ancestry as myelinating Sc but has a different fate during the immature Sc stage [31].
As previously mentioned, Remak Sc cells will engulf any tiny axons (less than 1 μm in diameter) that remain after radial sorting, forming Remak bundles (Figure 1). The outcome of the choice between promyelinating and Remak Sc is still unknown, though. Although NRG1-III signalling is not the only signalling pathway necessary for fate specification [31], axonal NRG1-III is crucial for Remak bundle formation [32]. Moreover, different gene expression profiles, such as transcription factors, cell adhesion molecules, and receptor alterations, are confirmed by myelinating Sc and Remak Sc [24]. The full ensheathing of every axon in the bundle, with the Remak Sc cell membrane separating axons from one another, is a characteristic of mature Remak bundles [31]. There are parallels and divergences between myelinating Sc and Remak Sc maturation and maintenance. PI-3 K/AKT-1 and Gpr126 signals are similar, but Neuropathy Target Esterase (NTE) expression is different [31-33].
Brief comments on non-myelinating terminal Schwann cells
The Neuromuscular Junction (NMJ) in skeletal muscle and several sensory end-organ structures in skin, including free nerve endings, Meissner corpuscles, Pacinian corpuscles, and hair follicles, are among the peripheral nerve axons that contain terminal Sc [34].
Similar to myelinating Sc and Remak Sc, terminal Sc originates from the neural crest via a Sc precursor lineage. However, it is still unclear what variables influence the specification of terminal Sc cell fate [16].
However, terminal Sc develops and matures in tandem with the maturity of the end-organ structure. Because of their connection to ALS, some scientists have concentrated on NMJ terminal Sc, also known as perisynaptic Sc, for the rest of this review. It is also commonly known that, as illustrated graphically in Figure 1 and corroborated by the previously cited researchers [35], chemical synapses between myelinated motor neurone axons and skeletal muscle fibres are made up of synaptic clefts, presynaptic axon terminal boutons, and postsynaptic muscle end plates.
We postulated that the proteins promote synaptic vesicle docking and fusion, and that terminal boutons with active zones packed with Ca2+ channels and synaptic vesicles laden with Acetylcholine (ACh) facilitate IS symptoms and indicators.
However, excess ACh generated in response to action potentials is catabolised by acetylcholinesterase found in the synaptic cleft.
According to other researchers, genes involved in phagocytosis, cell adhesion, and extracellular matrix synthesis were enriched in the gene expression profiles of perisynaptic Sc [36]. Since perisynaptic Sc is also necessary for appropriate NMJ transmission, including regulating synaptic plasticity and sensing synaptic activity, we postulated that IS may impact the presynaptic membrane's typical neurophysiological mechanism of action. Nevertheless, little is known about the mechanics underlying these activities [37].
Furthermore, we postulated that perisynaptic Sc, which act as tracts for regenerating axons to expand along in order to facilitate reinnervation of original synaptic locations, plays a critical role during reinnervation following nerve damage caused by hyperexcitability of the axon in cases of IS [38,39].
Brief comments on satellite glial cells
We graphically represented Satellite glia (Sg) in Figure 1. Sg are flattened cells that tightly wrap around NC bodies in the autonomic and sensory ganglia. The Dorsal Root Ganglia (DRG) house the cell bodies of all sensory neurones that innervate targets below the neck. Nonetheless, the sensory neurones innervating the skull are part of the trigeminal and nodose ganglia [40]. There is a positive correlation between soma size and the number of satellite glial cells per neurone; in mice, estimations of 4-12 satellite glia per DRG neurone have been recorded [41]. The neural crest gives rise to Satellite glial (Sg) cells via a Sc precursor lineage, which includes both myelinating and non-myelinating Sc [42].
Omics research shows that Sg cells contain markers such as Sc precursors and their CNS equivalent, Astrocytes (As), despite the fact that the gene expression and signalling pathways that govern Sg cell fate specification are yet unclear. Another shared feature between Sg and As is their capacity to be expressed in response to inflammation and injury. The Sg-neuronal soma unit is enclosed by a single basal lamina. Gap junctions allow the Sg in a single unit to regulate synaptic transmission, neuronal homeostasis, and connection [16].
Glial Fibrillary Acidic Protein (GFAP) overexpression, gap junction connectivity, and Sg expression all rise in response to inflammation and damage. Neuropathic pain is caused by the production of proinflammatory cytokines by activated satellite glia, which raise excitability and cause the surrounding neurone to fire [16]. Similar to fate specification, nothing is understood about Sg's maturation and maintenance.
Brief comments on myelin sheath disorder in Charcot Marie-Tooth disease (CMT)
The most prevalent hereditary neuropathy is Charcot Marie-Tooth disease (CMT), a collection of genetic illnesses that impact the nerves that connect the Central Nervous System (CNS) to the rest of the body.
Gene mutations are modifications in a gene's DNA that can change how the gene works. More than one hundred genes are linked to CMT that can affect the myelin sheath, the axon or both.
Like IS, CMT commonly affects nerves that control a person’s muscles, leading to muscle weakness and NP mainly in type 1 presentation. In some sporadic cases of IS the clinical features of the NP cannot be easily differentiated from the CMT. In Table 1 we summarized some updated information on CMT1 caused by genes damaging the Sc.
| Gene | Gene function | CMT1 subtype | Mutation type and location | CMT severity | |
| CMT1 is caused by the mutation of genes affecting myelinating Schwann cell. | Lipopolysaccharide Induced Tumor Necrosis Factor-alpha factor (LITAF/SIMPLE) | Recruitment of ESCRT components for endosomal trafficking and signaling | CMT1C | Point mutations, primarily localized to the C-terminal cysteine-rich domain | Classic CMT |
| Myelin architecture regulation (undefined role in cell adhesion), Schwann cell proliferation and survival | CMT1A | Gene duplication | Classic CMT | Myelin architecture regulation (undefined role in cell adhesion), Schwann cell proliferation and survival | |
| Peripheral Myelin Protein 22 (PMP22) | Myelin architecture regulation (undefined role in cell adhesion), Schwann cell proliferation and survival | CMT1A | Gene duplication | Classic CMT | |
| Myelin Protein Zero (MPZ/P0) | Adhesion protein required for compaction of myelin lamellae | CMT1B | Numerous point mutations identified, primarily localized to the extracellular domain | Severe or classic CMT | |
| Early Growth Response 2 (EGR2/Krox20) |
Master transcription factor controlling Schwann cell myelination | CMT1D | Point mutations, primarily localized in the Zinc finger domains |
Severe or classic CMT | |
| Neurofilament light polypeptide (NEFL) | CMT2E | Point mutations, localized throughout the protein | Severe or classic CMT, hearing loss | ||
| Peripheral Myelin Protein 2 (PMP2) | Myelin sheath stiffness, membrane stacking, and lipid transfer | CMT1G | Point mutations, primarily localized to the fatty acid binding pocket | Classic CMT | |
| Fibulin 5 gene (FBLN5) | Extracellular matrix protein | CMT1H | Point mutations, few identified to date | Classic or mild CMT | |
| Polymerase III, RNA, subunit B (POLR3B) | Subunit of RNA polymerase transcribing non-coding RNA, also involved in RNA processing and translation | CMT1I | Point mutations, few identified to date | Severe CMT, also intellectual disability, spasticity, and ataxia | |
| Inositol 1,4,5-Trisphosphate Receptor, type 3 (ITPR3) | Receptor for Inositol Triphosphate (IP3), intracellular calcium release | CMT1J | Point mutations, few identified to date | Severe or classic CMT | |
| Modified from the original one | Gap Junction Protein Beta 1 (GJB1/Cx32) | Gap junction hemichannel involved in signaling molecules throughout myelin and transporting metabolites | CMT1X | Numerous point mutations identified, localized throughout the protein | Classic CMT, males more affected than females, more frequent hand deformities and CNS symptoms |
Table 1: Summarized some updated information on CMT1 caused by genes damaging the Sc
Brief comments on Schwann cell dysfunction in IS
Based on Sc's documented essential functions in PNS, such as insulators to promote saltatory conduction, axonal cytoskeleton, and myelinating, Sc also organises the node of Ranvier, provides axons with metabolic and trophic support, and protects axons from insults [43], we hypothesised that Sc is intensely involved in the pathogenesis of hyperexcitability of the peripheral nerves.
As other authors have reported, Remak Sc, an immunocompetent cell, supplies the axons' primary trophic, metabolic, and immunological support [44].
Terminal Sc are generally included in end-organ structure maintenance, development, and plasticity, while perisynaptic Sc at NMJs are involved in synaptic neurotransmission [16]. Sg cells exert various effects on their surrounding neuronal soma, including protection from insults, metabolic support, and the regulation of synaptic transmission [40]. Thus, it is not unexpected that IS, which is brought on by peripheral nerve hyperexcitability and results in dysfunction of myelinating Sc [45], terminal Sc [46], and Sg [16], has been found to induce severe PNS functional impairment that adversely affects a patient's quality of life.
Brief comments on neuropathic pain related to Isaacs syndrome
Some authors define pain as "an unpleasant emotional and sensory experience resembling or associated with potential or actual tissue damage" [47]. Identifying painful stimuli is essential for a species' survival and well-being, as it prevents the development of injured tissue and compromises its long-term functioning.
The perception of nociception involves all the physiological processes integrating real tissue damage or a painful stimulus to signal potential. Therefore, pain perception relies on the sensitivity of altered tissue, which is projected to CNS structures before being integrated into memory and emotional networks. Nociceptive stimuli come in three varieties: Thermal, mechanical, and chemical. If a certain type of stimulation raises a sensory receptor's activation threshold, it is detrimental [47]. Similar to sensory NC, the nociceptive signal is triggered by nociceptors, which send a pain signal to the Central Nervous System (CNS). These nociceptors, sometimes referred to as Aδ or C fibres, are typically unmyelinated or poorly myelinated. We postulated that in IS instances, a membrane depolarisation known as a receptor potential triggers the transmission of pain signals at the nociceptors' peripheral nerve endings. Action potentials generated by this depolarisation in these patients transfer information from the peripheral terminals to the spinal cord's dorsal horn. From here, it is integrated and transmitted to the brain via the spinothalamic tracts to the primary sensory cortex in the parietal lobe.
The Transient Receptor Potential (TRP) channel family gathers throughout species receptors mainly permeable to Na+ and Ca2+ ions and then divided into six subfamilies: Canonical (TRPC), Ankyrin (TRPA), Melastatin (TRPM), Polycystin (TRPP), Vanilloid (TRPV), and Mucolipin (TRPML) [47]. Some of them are activated in nociceptors and thus involved in the transduction of a nociceptive stimulus. Their activation in the sensory afferents induces membrane depolarisation, which ultimately generates a painful stimulation in the CNS [47].
Neuropathic Pain (NP) is a chronic condition that results from dysfunctional central and peripheral pain conduction pathways, characterized by chronic spontaneous pain, hyperalgesia, and allodynia, often perceived as a refractory pain syndrome [48].
The central part pathway includes the posterior horn of the spinal cord, which is a gray matter that receives sensory information from the peripheral sensory receptor at the periphery and send it to the sensory brain cortex via ascending pathways.
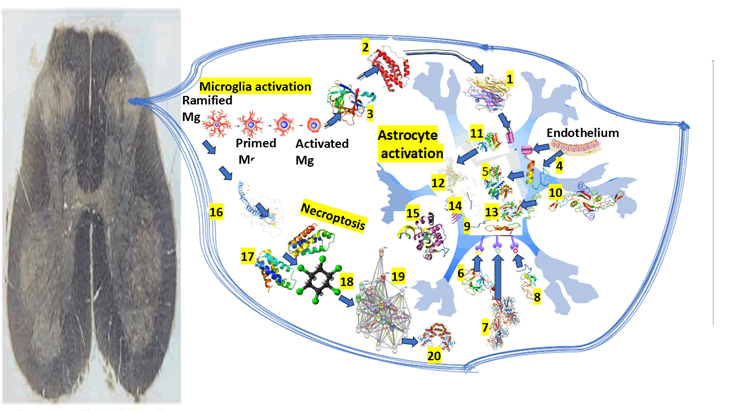
The graphical representation of our hypotheses related to the pathophysiology of NP in IS involving the Mg/As expression in the posterior horn of the SC can be seen in Figure 2.
Figure 2: Graphical representation of the central mechanism of NP
Note: 1) TNF alpha, 2) IL-6, 3) IL-1b, 4) Sp1 is a protein which acts as a transcriptional activator, regulating gene expression by binding to DNA and modulating whether a gene is turned off or on. It plays an important role in several biological processes including apoptosis, cell growth, and differentiation. 5) Cyclin D1 is also a protein that plays a vital role in modulating cell cycle progression. 6) Fibroblast Growth Factor (FGF) is a protein that stimulate the proliferation mainly of endothelial cells and that promote angiogenesis. 7) Basic Fibroblast Growth Factor (bFGF), is a signaling protein encoded by the FGF2 gene, involved in various biological processes including tumor growth, angiogenesis, and wound healing. 8) Epidermal Growth Factor (EGF) is a protein that stimulates cell growth and differentiation by binding to its receptor. 9) Notch1 is a transmembrane receptor protein encoded by the NOTCH1 gene. 10) Transforming Growth Factor-beta (TGF-β) is a multifunctional cytokine that plays a crucial role in cell growth, apoptosis, differentiation, and is involved in embryonic development, immune responses and wound healing. 11) Janus Kinase (JAK) inhibitors used to treat chronic inflammatory disorders and increase the risk of major cardiovascular diseases like heart attack or stroke, blood clots in the lungs, cancer, serious infections and death when compared with TNF alpha inhibitors. 12) STAT3, Signal Transducer and Activator of Transcription 3, is a protein that regulates gene expression by binding to DNA. It plays a crucial role in immune responses, cell growth, and differentiation, and it is implicated in the development of cancer. 13) SMAD is an intracellular protein that plays a crucial role in regulating growth, cell development, and other biological processes. 14) OLIG2, or Oligodendrocyte Transcription Factor 2, is crucial for the development and function of the CNS, particularly in the specification and differentiation of astrocytes and oligodendrocytes. 15) ATP, or adenosine triphosphate, is the main energy source of cells. 16) TSC2, also named tuberin, is a tumor suppressor gene that encodes a protein crucial for controlling cell growth and division associated to associated with tuberous sclerosis complex. 17) mTOR, or mechanistic target of rapamycin, is a protein kinase that plays a vital role in regulating metabolism, cell growth, and survival. 18) HCH. 19) RIP3, or receptor-interacting protein kinase 3, is a key protein involved in programmed cell death necroptosis, a pathway characterized by cell membrane rupture and inflammation. 20) p62, also known as sequestosome 1, is a multifunctional protein involved in cellular homeostasis, autophagy, and signaling.
Dong et al., also found that following chronic constriction injury surgery, the mammalian Target of Rapamycin (mTOR) increased in As and As' expression within the spinal cord. This point is appropriated to highlight some aspects related to the mTOR, which is a member of the family protein kinases encoded by the MTOR gene with the capacity to regulate several cellular processes such as cell survival, protein synthesis, cell growth, cell proliferation, cell motility, autophagy/apoptosis (by participating in multiple signalling pathways in the body) transcription, expression of insulin receptors and insulin-like growth factor 1 receptor; mTORC2 has also been involved in the maintenance and control of the actin cytoskeleton; mTOR signalling pathway is also implicated with arthritis, insulin resistance, cancer, osteoporosis among many other disorders.
We hypothesized that NP in patients presenting IS, the neuroinflammation and hyperalgesia brought on by CCI were reversed by pharmacologically inhibiting mTOR. If not, the downregulation of spinal glutamate metabolism related protein activity may be restored by astrocytic mTOR knockdown, highlighting the critical function of mTOR in regulating this pathway.
According to the same authors [48], intrathecal TSC2 shRNA injection resulted in overexpression of mTOR, causes an upregulation of Receptor-Interacting Protein 3 (RIP3), which can be applied to cases of IS.
On the other hand, pharmacological inhibition blockage of RIP3 might eliminate the mTOR-induced As activation while not modulating mTOR activation, which can control the RIP3 expression in As through ITCH-mediate by autophagy-dependent degradation. Despite a few effective treatments for NP, we hypothesized that the results of drug therapy of NP in cases with IS will improve if we find all the necessary knowledge to modulate the link between mTOR and RIP3 producing As expression, leading to a proper peripheral and central sensitization. The sustainability of chronic NP in cases with IS should be considered because of an enhanced neuronal reactivity in central pain pathways after painful damage/NI, which drives central sensitization.
However, activating Mg and As causes chemokines and proinflammatory cytokines to be released, which exacerbates NI. It is corroborated by other researchers who have shown how As contributes to the formation and upkeep of NP [49-51]
The above postulate is supported by Fang et al., who show that inhibiting astrocyte activity in the spinal cord reduces pain sensitivity [52].
Unfortunately, after completing our systematic review on this issue, we could not find the appropriate information to determine how mTOR is involved in As activation leading to NP. Nonetheless, several scientists have confirmed that mTOR inhibition reduces NI and demyelination in globoid cell leukodystrophy [53].
We hypothesized that in patients with IS, RIP3 is activated at a high level in reactive As, and the activation of RIP3 during cellular stress induces the NI response.
We previously documented two protein degradation pathways, and the autophagy-lysosome and ubiquitin proteasome systems were explained mainly [54].
As mentioned before, NP is induced by As mTOR. Due to a lack of degradation, we believe that RIP3 can remarkably accumulate in the As, releasing proinflammatory elements; therefore, NI induces the enhancement of NP, increasing neuronal expression.
We also hypothesized that in cases presenting IS, NP is produced when the NF-κB signalling pathway in astrocytes is activated, and when it builds up in significant quantities, it damages neurones, as has been documented by other authors under different conditions [54].
However, other authors have documented that under different conditions presenting chronic pain, activated As become less able to absorb the large amount of glutamate released from other As and neurons [48]. Moreover, under normal circumstances, GLT-1, the glutamate transporter proteins, and GS mediate this uptake [55].
In cases of IS presenting NP, it might be related to a decreased mTOR in As inhibiting glutamate release in the CNS as part of the regulation of glutamate metabolism by mTOR.
As documented by the previously cited investigators, c-fos (a marker for neuronal activity after nociceptive stimulation) expressed in the nucleus of damaged sensory neurons is downregulated by As mTOR and NP occur via induction of RIP3 [48]. Therefore, RIP3 maintains and produces NI and NP and can be targeted for pain management [56].
As a result, we believe that RIP3 inhibitors administered intrathecally may reduce NP in IS patients.
Additionally, we think that macrophage infiltration can result from Paclitaxel (also known as Taxol), which inhibits the growth and proliferation of cancerous cells, leading to the release of IL-1β and TNF-α in the posterior root ganglion DRG, and can cause NP through RIP3 serving as supporting test but not recommendable because its bioethical implications. Nevertheless, there is no doubt that RIP3, reactive As and NP are very strongly interrelated, which is supported by the fact that RIP3 is upregulated in animals approached by CCI surgery, while an inhibition of mTOR decreased RIP3 expression [48]. Therefore, makes sense to consider that mTOR can induce As activation and release proinflammatory elements by increasing RIP3 activity.
Additional brief comments on drug therapy
The scanty number of therapeutic drug options and their poor efficacy make managing NP in IS a big challenge. Most current drug therapy has been developed based on clinical observations and limited experience rather than focusing on specific underlying mechanisms [57].
The biggest impediment to developing targeted drug therapy is the lack of knowledge on the neuropathophysiological mechanisms of NP [58].
Notwithstanding, the results of investigations on glial cells contribute to the best understanding of the therapeutic choices for NP [59].
Giving a proper relief of NP is hampered by the absence of specificity of research techniques to provide an adequate effect from glial cell inhibitors [60,61]
Recently, some investigators [48] used a specific type of virus-containing cell promoters to achieve a particular delivery of a gene to As. However, their results have not been administered to human populations despite their ability to identify the exact elements responsible for As expression and neuronal effects, offering vital new information in this area. Additionally, certain medication therapies that target the mTOR-RIP3 pathway function by inhibiting neuronal activity and/or regulating overall discomfort [62].
Fortunately, there exists the potential for the development of highly safe and effective personalized drug therapy protocols [48].
The most recent contribution to the therapeutic management of the NP was reported by Xiuying He and collaborators [63], who documented the administration of Vof16 as a new therapeutic target for alleviating NP. They found that long noncoding RNAs (lncRNAs)- Vof16 (Vof16) plays a remarkable role in maintaining proper sensory function under healthy conditions and serves as a protective shield against Neuropathy (NP), particularly in cases of peripheral nerve injury.
Conclusion
We finally hypothesised that the forthcoming therapeutic drug should be based on the efficacy of modulating mTOR, a crucial signalling molecule involved in the expression of A1 astrocytes, central sensitisation in an NP, and the RIP3 model. As far we know, this is the first comprehensive review on NP/IS/therapeutic drug results. We hope that further investigations will provide the necessary information to support or reject our hypotheses focusing on mTOR and RIP3 as potential drug therapy.
Author Contributions
Both investigators have read and agreed to the published version of the manuscript.
Conflict of Interest Statement
The authors declare they have no conflicts of interest.
Funding Information
The authors received no funds to perform the present research.
Ethics Statement
The study was conducted using the principles of the Hel sinki Declaration, the Italian and US privacy and sensitive data laws, and the internal regulations for retrospective studies of the Otolaryngology Section at Padova University and Brescia University (Italy).
Informed Consent Statement
We obtained the informed consent from the case involved in the study.Data Availability Statement
The corresponding author will make the raw data supporting this article's conclusions available upon request.
Acknowledgments
The authors thank Dr. Sibi Joseph for his participation in managing this patient and for completing the summary of the case presentation.References
- H. Isaacs, A syndrome of continuous muscle-fibre activity, J Neurol Neurosurg Psychiatriy, 24(1961):319-325.
[Crossref] [Google Scholar] [PubMed]
- H. Foyaca-Sibat, L. Ibanez-Valdes, Issacs' syndrome successfully treated with phenytoin at low doses, Internet J Neurol, 1(2001).
- Tani, S. Mizutani, M. Watanabe, T. Irie, K. Masaki, et al. Oral management for a patient with trismus accompanied by Isaacs’ syndrome: A case report, BMC Oral Health, 24(2024):716.
[Crossref] [Google Scholar] [PubMed]
- P. Sarraf, M. Shafie, G. Farahmand, M. Mayeli, M. Shahbazi, et al. Isaacs’ syndrome: Clinical and paraclinical perspectives in a series of cases, Curr J Neurol, 23(2024):1-14.
[Crossref] [Google Scholar] [PubMed]
- Orphanet. Issacs syndrome, Eur J Neurol, 2013.
- P.P. Tripathi, S. Kumari, N. Prabhat, D.S. Lamba, R. Hans, et al. Effectiveness of therapeutic plasma exchange in the case of Isaacs syndrome rare neurological disorder, Asian J Transfus Sci, 17(2023):117–120.
[Crossref] [Google Scholar] [PubMed]
- E.A. Jaben, J.L. Winters, Plasma exchange as a therapeutic option in patients with neurologic symptoms due to antibodies to voltage-gated potassium channels: A report of five cases and literature review, J Clin Apher, 27(2012):267–273.
[Crossref] [Google Scholar] [PubMed]
- H. Isaacs, G. Frere, Syndrome of continuous muscle fibre activity. Histochemical, nerve terminal and end-plate study of two cases, S Afr Med J, 48(1974):1601–1607.
[Google Scholar] [PubMed]
- P. Sarraf, M. Shafie, G. Farahmand, M. Mayeli, M. Shahbazi, et al. Isaacs syndrome: Clinical and paraclinical perspectives in a series of cases, Curr J Neurol, 23(2024):1–14.
[Crossref] [Google Scholar] [PubMed]
- P.A. Barber, N.E. Anderson, A. Vincent, Morvan's syndrome associated with voltage-gated K+ channel antibodies, Neurology, 54(2000):771–772.
[Crossref] [Google Scholar] [PubMed]
- K. Arimura, Y. Sonoda, O. Watanabe, T. Nagado, A. Kurono, et al. Isaacs syndrome as a potassium channelopathy of the nerve, Muscle Nerve Suppl, 11(2002):S55–S58.
[Crossref] [Google Scholar] [PubMed]
- Vincent, P. Pettingill, R. Pettingill, B. Lang, R. Birch, et al. Association of leucine-rich glioma inactivated protein 1, contactin-associated protein 2, and contactin 2 antibodies with clinical features and patient-reported pain in acquired neuromyotonia, JAMA Neurol, 75(2018):1519–1527.
[Crossref] [Google Scholar] [PubMed]
- S.B. Park, R. Thurbon, M.C. Kiernan, Isaacs’s syndrome: The frontier of neurology, psychiatry, immunology and cancer, J Neurol Neurosurg Psychiatry, 91(2020):1243–1244.
[Crossref] [Google Scholar] [PubMed]
- Falace, P. Striano, F. Manganelli, A. Coppola, S. Striano, et al. Inherited neuromyotonia: A clinical and genetic study of a family, Neuromuscul Disord, 17(2007):23–27.
[Crossref] [Google Scholar] [PubMed]
- S.S. Rana, R.S. Ramanathan, G. Small, B. Adamovich, Paraneoplastic Isaacs' syndrome: A case series and literature review, J Clin Neuromuscul Dis, 13(2012):228–233.
[Crossref] [Google Scholar] [PubMed]
- K.R. Moss, S. Saxena, Schwann cells in neuromuscular disorders: A spotlight on amyotrophic lateral sclerosis, Cells, 14(2025):47.
- C.B. Reed, M.L. Feltri, E.R. Wilson, Peripheral glia diversity, J Anat, 241(2022):1219-1234.
[Crossref] [Google Scholar] [PubMed]
- G.M. Manzano, L.M. Giuliano, J.A. Nobrega, A brief historical note on the classification of nerve fibers, Arq Neuropsiquiatr, 66(2008):117–119.
[Crossref] [Google Scholar] [PubMed]
- K.R. Jessen, R. Mirsky, Schwann cell precursors; multipotent glial cells in embryonic Nerves, Front Mol Neurosci, 12(2019):69.
[Crossref] [Google Scholar] [PubMed]
- J. Buchstaller, L. Sommer, M. Bodmer, R. Hoffmann, U. Suter, et al. Efficient isolation and gene expression profiling of small numbers of neural crest stem cells and developing Schwann cells, J Neurosci, 24(2004):2357–2365.
[Crossref] [Google Scholar] [PubMed]
- M. D’Antonio, D. Michalovich, M. Paterson, A. Droggiti, A. Woodhoo, et al. Gene profiling and bioinformatic analysis of Schwann cell embryonic development and myelination, Glia, 53(2006):501–515.
[Crossref] [Google Scholar] [PubMed]
- M.L. Feltri, Y. Poitelon, S.C. Previtali, How Schwann cells sort axons: New Concepts, Neuroscientist, 22(2016):252–265.
[Crossref] [Google Scholar] [PubMed]
- J.A. Pereira, F. Lebrun-Julien, U. Suter, Molecular mechanisms regulating myelination in the peripheral nervous system, Trends Neurosci, 35(2012):123–134.
[Crossref] [Google Scholar] [PubMed]
- J. Salzer, M.L. Feltri, C. Jacob, Schwann cell development and myelination, Cold Spring Harb Perspect Biol, 16(2024):a041360.
[Crossref] [Google Scholar] [PubMed]
- C. Taveggia, Schwann cells-axon interaction in myelination, Curr Opin Neurobiol, 39(2016):24–29.
[Crossref] [Google Scholar] [PubMed]
- C. Taveggia, G. Zanazzi, A. Petrylak, H. Yano, J. Rosenbluth, et al. Neuregulin-1 type III determines the ensheathment fate of axons, Neuron, 47(2005):681–694.
[Crossref] [Google Scholar] [PubMed]
- Y. Poitelon, C. Lopez-Anido, K. Catignas, C. Berti, M. Palmisano, et al. YAP and TAZ control peripheral myelination and the expression of laminin receptors in Schwann cells, Nat Neurosci, 19(2016):879–887.
[Crossref] [Google Scholar] [PubMed]
- N. Tricaud, Myelinating Schwann cell polarity and mechanically-driven myelin sheath elongation, Front Cell Neurosci, 11(2017):414.
[Crossref] [Google Scholar] [PubMed]
- T.L. Brown, W.B. Macklin, The actin cytoskeleton in myelinating cells, Neurochem Res, 45(2020):684–693.
[Crossref] [Google Scholar] [PubMed]
- S. Atanasoski, S.S. Scherer, E. Sirkowski, D. Leone, A.N. Garratt, et al. ErbB2 signaling in Schwann cells is mostly dispensable for maintenance of myelinated peripheral nerves and proliferation of adult Schwann cells after injury, J Neurosci, 26(2006):2124–2131.
[Crossref] [Google Scholar] [PubMed]
- B.L. Harty, K.R. Monk, Unwrapping the unappreciated: Recent progress in remak Schwann cell biology, Curr Opin Neurobiol, 47(2017):131–137.
[Crossref] [Google Scholar] [PubMed]
- F.R. Fricker, N. Zhu, C. Tsantoulas, B. Abrahamsen, M.A. Nassar, et al. Sensory axon-derived neuregulin-1 is required for axoglial signaling and normal sensory function but not long-term axon maintenance, J Neurosci, 29(2009):7667–7678.
[Crossref] [Google Scholar] [PubMed]
- J. McFerrin, B.L. Patton, E.R. Sunderhaus, D. Kretzschmar, NTE/PNPLA6 is expressed in mature Schwann cells and is required for glial ensheathment of Remak fibers, Glia, 65(2017):804–816.
[Crossref] [Google Scholar] [PubMed]
- R.L. Hastings, G. Valdez, Origin, identity, and function of terminal Schwann cells, Trends Neurosci, 47(2024):432–446.
[Crossref] [Google Scholar] [PubMed]
- P.M. Rodriguez Cruz, J. Cossins, D. Beeson, A. Vincent, The neuromuscular junction in health and disease: Molecular mechanisms governing synaptic formation and homeostasis, Front Mol Neurosci, 13(2020):610964.
[Crossref] [Google Scholar] [PubMed]
- R. Castro, T. Taetzsch, S.K. Vaughan, K. Godbe, J. Chappell, et al. Specific labeling of synaptic schwann cells reveals unique cellular and molecular features, eLife, 9(2020):e56935.
[Crossref] [Google Scholar] [PubMed]
- T.W. Gould, C.P. Ko, H. Willison, R. Robitaille, Perisynaptic Schwann cells: Guardians of neuromuscular junction integrity and function in health and disease, Cold Spring Harb Perspect Biol, 10(2024):a041362.
[Crossref] [Google Scholar] [PubMed]
- S. Negro, F. Lessi, E. Duregotti, P. Aretini, M. La Ferla, et al. CXCL12alpha/SDF-1 from perisynaptic Schwann cells promotes regeneration of injured motor axon terminals, EMBO Mol Med, 9(2017):1000–1010.
- H. Kang, L. Tian, M. Mikesh, J.W. Lichtman, W.J. Thompson, Terminal Schwann cells participate in neuromuscular synapse remodeling during reinnervation following nerve injury, J Neurosci, 34(2014):6323–6333.
[Crossref] [Google Scholar] [PubMed]
- M. Hanani, D.C. Spray, Emerging importance of satellite glia in nervous system function and dysfunction. Nat Rev Neurosci, 21(2020):485–498.
[Crossref] [Google Scholar] [PubMed]
- McGinnis, R.R. Ji, The similar and distinct roles of satellite glial cells and spinal astrocytes in neuropathic pain, Cells, 12(2023):965.
[Crossref] [Google Scholar] [PubMed]
- D. George, P. Ahrens, S. Lambert, Satellite glial cells represent a population of developmentally arrested Schwann cells, Glia, 66(2018):1496–1506.
[Crossref] [Google Scholar] [PubMed]
- K.R. Moss, T.S. Bopp, A.E. Johnson, A. Hoke, New evidence for secondary axonal degeneration in demyelinating neuropathies, Neurosci Lett, 744(2021):135595.
[Crossref] [Google Scholar] [PubMed]
- E. Ydens, G. Lornet, V. Smits, S. Goethals, V. Timmerman, et al. The neuroinflammatory role of Schwann cells in disease, Neurobiol Dis, 55(2013):95–103.
[Crossref] [Google Scholar] [PubMed]
- S.S. Scherer, J. Svaren, Peripheral Nervous System (PNS) myelin diseases, Cold Spring Harb Perspect Biol, 16(2024):a041376.
[Crossref] [Google Scholar] [PubMed]
- K.B. Santosa, A.M. Keane, A. Jablonka-Shariff, B. Vannucci, A.K. Snyder-Warwick, Clinical relevance of terminal Schwann cells: An overlooked component of the neuromuscular junction, J Neurosci Res, 96(2018):1125–1135.
[Crossref] [Google Scholar] [PubMed]
- F. Beignon, M. Notais, S. Diochot, A. Baron, Z. Fajloun, et al. Neurotoxins acting on TRPV1-building a molecular template for the study of pain and thermal dysfunctions, Toxins (Basel), 17(2025):64.
- B. Dong, D. Li, S. Song, N. He, S. Yue, et al. MTOR promotes astrocyte activation and participates in neuropathic pain through an upregulation of RIP3, Neurochem Res, 50(2025):93.
[Crossref] [Google Scholar] [PubMed]
- Z.J. Zhang, B.C. Jiang, Y.J. Gao, Chemokines in neuron-glial cell interaction and pathogenesis of neuropathic pain, Cell Mol Life Sci, 74(2017):3275–3291.
[Crossref] [Google Scholar] [PubMed]
- C. Sommer, M. Leinders, N. Uceyler, Inflammation in the pathophysiology of neuropathic pain, Pain, 159(2018):595–602.
[Crossref] [Google Scholar] [PubMed]
- R.R. Ji, C.R. Donnelly, M. Nedergaard, Astrocytes in chronic pain and itch, Nat Rev Neurosci, 20(2019):667–685.
[Crossref] [Google Scholar] [PubMed]
- Y. Fang, Astrocytic phosphatase and tensin homolog deleted on chromosome 10 regulates neuropathic pain by facilitating 3-hydroxy-3-methylglutaryl-CoA reductase-dependent cholesterol biosynthesis, Pain, 163(2022):e1192–e1206.
[Crossref] [Google Scholar] [PubMed]
- D.S. Lin, Y.W. Huang, T.H. Lee, L. Chang, Z.D. Huang, et al. Rapamycin alleviates protein aggregates, reduces neuroinflammation, and rescues demyelination in globoid Cell leukodystrophy, Cells, 12(2023):993.
[Crossref] [Google Scholar] [PubMed]
- M.A. Wheeler, M. Jaronen, R. Covacu, S.E. Zandee, G. Scalisi, et al. Environmental control of astrocyte pathogenic activities in CNS inflammation, Cell. 176(2019):581-596.
[Crossref] [Google Scholar] [PubMed]
- L. Lisi, P. Navarra, D.L. Feinstein, C. Dello Russo, The mTOR kinase inhibitor rapamycin decreases iNOS mRNA stability in astrocytes, J Neuroinflammation, 8(2011):1.
- Y. Duan, Q. Li, Y. Zhou, S. Chen, Y. Li, et al. Activation of the TNF-α-necroptosis pathway in parvalbumin-expressing interneurons of the anterior cingulate cortex contributes to neuropathic pain, Int J Mol Sci, 24(2023):15454.
[Crossref] [Google Scholar] [PubMed]
- N. Attal, D. Bouhassira, Translational neuropathic pain research, Pain, 160(2019):S23–S28.
[Crossref] [Google Scholar] [PubMed]
- K. Bannister, J. Sachau, R. Baron, A.H. Dickenson, Neuropathic pain: Mechanism-based therapeutics, Annu Rev Pharmacol Toxicol, 60(2020):257–274.
[Crossref] [Google Scholar] [PubMed]
- R.R. Ji, A. Chamessian, Y.Q. Zhang, Pain regulation by non-neuronal cells and inflammation, Sci, 354(2016):572–577.
[Crossref] [Google Scholar] [PubMed]
- X. Fan, R. Chu, X. Jiang, LPAR6 participates in neuropathic pain by mediating astrocyte cells via ROCK2/NF-κB signal pathway, Mol Neurobiol, 61(2024):8402–8413.
[Crossref] [Google Scholar] [PubMed]
- J.Y. Lee, C.S. Park, K.J. Seo, IL-6/JAK2/STAT3 axis mediates neuropathic pain by regulating astrocyte and microglia activation after spinal cord injury, Exp Neurol, 370(2023):114576.
[Crossref] [Google Scholar] [PubMed]
- P.T. Hansson, A.H. Dickenson, Pharmacological treatment of peripheral neuropathic pain conditions based on shared commonalities despite multiple etiologies, Pain, 113(2005):251–254.
[Crossref] [Google Scholar] [PubMed]
- H. Xiuying, H.D. Mauki, X. Zhao, S. Dai, H. Yang, et al. Targeting LncRNAâVof16: A novel therapeutic strategy for neuropathic pain relief, CNS Neurosci Ther, 31(2025):e70241.
[Crossref] [Google Scholar] [PubMed]
Copyright: ©2025 Lourdes de Fatima Ibanez Valdes, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.