Research Article: Journal of Drug and Alcohol Research (2021) Volume 10, Issue 8
Levomethadone Safety Profile and Effectiveness in Subjects under Opioid Maintenance Treatment: An Observational, Prospective Study
Augusto Consoli1*, Paola Fasciani2, Emilio Vanoli3,4 and Marco Riglietta52Department of UOC Servizi Dipendenze, ASL Lanciano Vasto Chieti, Italy
3IRCCS Istituto San Giovanni di Dio Fatebenefratelli, Italy
4Department of Molecular Medicine, University of Pavia, Italy
5Dipartimento di Salute Mentale e Dipendenze, ASST Papa Giovanni XXIII, Italy
Augusto Consoli, Department of Clinica S, Caterina-GVM, Italy, Email: augusto.consoli.1@gmail.com
Received: 30-Jul-2021;Accepted Date: Aug 16, 2021; Published: 23-Aug-2021
Abstract
Purpose: To provide further data on the safety and effectiveness of levo- methadone after its long term use in a real world setting, an observational study involving opioid addicted patients undergoing maintenance treat- ment with levomethadone is ongoing in Italy (LEVOPROACT study). This work provides the results of the interim analysis after 2 years from the first site activation.
Methods: This is a prospective, observational, non-interventional, open ended study conducted in ten Italian centers and involving patients aged
≥18 years with a diagnosis of opioid addiction (ICD-10 F11.2), initiating or currently undergoing levomethadone maintenance treatment as for routine medical practice.
Results: Long term levomethadone therapy is effective in the treatment of opioid addiction, with a reduction in craving and opioid use, and a favor- able risk/benefit ratio.
Conclusion: Data collected in the present interim analysis are very en- couraging and support the good effectiveness and safety profile of levo- methadone therapy.
Keywords
Levomethadone; Opioid maintenance treatment; Methadone; Levomethadone long term treatment; Opioid dependence
Introduction
Opioid maintenance treatment, combined with psychosocial interventions, is the most common therapy for opioid dependence. This approach is supported by positive outcomes, such as treatment retention, reduced illicit opioid use and reduction of reported risk behavior, drug related harm, and mortality [1–3].
The counter side of this benefit is associated with the negative effects of many psychoactive drugs, including methadone, on electrocardiogram (ECG) QT interval and the related risk for life threatening arrhythmia, namely Torsades de Pointes (TdP). This aspect must be taken into account in the treatment of patients with addiction, in particular, due to the very frequent association of methadone therapy with many other psychotropic drugs, such as anxiolytics, neuroleptics and antidepressants, which can contribute to a prolongation of the QT interval.
Methadone is the most prescribed μ receptor agonist in European countries, received by over two thirds (69%) of addicted subjects treated with an agonist [4].
Methadone is a 1:1 racemic mixture of two enantiomers: the right handed enantiomer (S methadone, d methadone or dextromethadone) and the left handed enantiomer (R methadone, l methadone or levomethadone) [5]. These enantiomers have different pharmacokinetic and pharmacodynamic properties: since 1940, it is known that the pharmacological effects of racemic methadone therapy are primarily due to levomethadone [6,7].
Indeed, compared with dextromethadone, levomethadone has an approximately 10 times higher affinity for the μ opioid receptor [8]. In addition, levomethadone has a better safety profile compared with racemic methadone because it seems to be less involved in the alteration of the electrocardiographic QT interval [9–11]. Moreover, thanks to the binding stereoselectivity for the different cytochrome P450 isoforms [12,13], levomethadone presents a metabolic pathway able to guarantee lower interactions with drugs that act on the central nervous system, frequently co-administered with opioid agonist therapy and often causing QT interval prolongation [14].
Levomethadone has been administered for the maintenance programs in Germany since 1987, while in Italy, in 2015, it was approved as an agonist maintenance therapy for opioid drug dependence in adults, in combination with appropriate medical, social and psychosocial support [15].
Despite its long term use in some countries, to date, there are limited data regarding the use in the clinical practice of levomethadone on a European scale, specifically on long term treatment.
To provide further data on the safety and effectiveness of levomethadone after its long term use in a real world setting, an observational study involving opioid addicted patients undergoing maintenance treatment with levomethadone is ongoing among different Italian centers (LEVOPROACT study). The present work aims to provide the results of the interim analysis after 2 years of the first site activation (first cut off, March 2020).
Patients and Methods
Subjects and study design
This is a prospective, observational, non-interventional, open ended study involving ten Italian centers, located in Rome, Bergamo, Milan, Soverato, Avellino, Aversa, AltamuraAltamura, Savona, Trieste and Chieti.
Male and female patients aged ≥18 years with a diagnosis of opioid addiction (ICD-10 F11.2), initiating or currently undergoing levomethadone maintenance treatment as for routine medical practice and according to the approved SmPC were screened for the study.
Patients who fulfill any of the following criteria were excluded: inability to understand study procedures, any contraindication stated in the SmPC for the administration of levomethadone according to the investigator’s judgment, patients currently participating in any other clinical trial.
Data were collected in accordance with routine procedures and according to the following timeline: baseline (visit 1 – V1, day 0), after 30 days (visit 2 – V2, day 30 ± 10), after 90 days (visit 3 – V3, day 90 ± 10) and after 180 days (visit 4 – V4, day 180 ± 15). A follow up (FU) visit was scheduled for day 360 ± 15. During V1, the investigator delivered to the patient a diary (patient’s substance use diary) to collect information about the recent history of substance use (heroin, buprenorphine, cocaine). At every visit, the investigator verified the collected information.
The study is divided into two different consecutive parts (Figure 1):
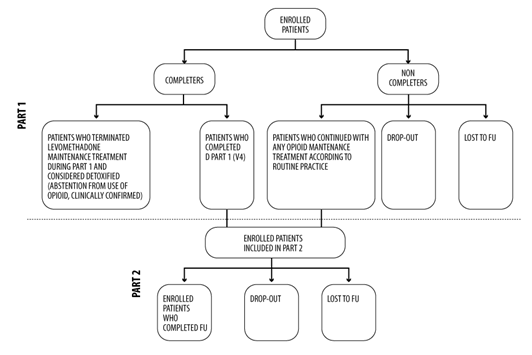
Figure 1: Flowchart of the study design.
-Part 1 (from Informed Consent Form (ICF) signature up to the last data collected at V4): the patients are in levomethadone maintenance treatment according to clinical practice.
-Part 2 (from V4 up to FU visit): patients are in the opioid maintenance treatment with any drug (including levomethadone) according to clinical practice (completer patients). A patient that did not complete Part 1 of the study but continues with any other opioid maintenance treatment according to routine practice has been included in Part 2 of the study (non-completer patients).
The maximum duration in the study for each patient is 405 days (from ICF signature until patient’s FU visit).
Patients or legal guardian, when applicable, must provide their written informed consent to participate in the study.
The study was conducted in compliance with the study protocol, the current revision of the Declaration of Helsinki (Fortaleza, Brazil, 2013), current Good Clinical Practice (ICH-GCP), and with local regulation for observational studies conduction. The ethics committee of all participant centers reviewed and approved the study protocol and the informed consent form before any subject was enrolled. ClinicalTrials.gov Identifier: NCT03685162.
Study measures
This first interim analysis was performed on Part 1 of the Study (V1–V4). Only patients with validated data of Part 1 visits and/or have undergone the end of treatment visit at the cut-off date (31 March 2020) were considered.
Safety
The primary aim of the study focused on the safety of levomethadone and was based on the assessment of the following variables: adverse drug reactions (ADRs), QTc prolongation at 12 lead ECG and laboratory examination, where available. In this interim analysis, only the initial ADR was analyzed (intended as an ADR which is not the result of a change of an already existing ADR). The analysis of QTc prolongation and laboratory examination was carried out only on patients with data assessed at both V1 and V4. A dedicated software (AMPS, Montichiari) was used to obtain automatic measures of QT interval corrected for heart rate using Bazett’s formula. All readings were then reviewed by an independent cardiologist blind to the patient’s status.
Secondary aims
Effectiveness: The effectiveness of levomethadone maintenance treatment was evaluated based on the following parameters:
1. Addiction Severity Index: evaluated with a 0–9 rating scale for the following domains: medical status, employment/support status, alcohol, drugs, legal status, family/social status and psychic status [16-18].
2. Craving: evaluated on a Visual Analogue Scale (VAS) from 0 to 100.
3. Quality of life: the SF-12 quality of life questionnaire, an abbreviated version of the SF-36 Health Related Quality of Life questionnaire, was used. The evaluation referred to the Physical Component Summary (PCS-12) and Mental Component Summary (MCS- 12), with the scores ranging from 0 (worst score) to 100 (best score) [19].
4. Catabolites in urine (heroin, methadone, buprenorphine, cocaine).
5. Patient’s substance use diary.
6. Retention in treatment rate: the proportion of patients assuming levomethadone in Part 1 of the study.
All the above mentioned analyses were carried out only on patients with data assessed at both V1 and V4.
Other measures
Levomethadone exposure (computed from levomethadone first intake at study entry to the last visit date), the blood level of levomethadone, the number of abnormalities in physical examinations, in vital signs, and ECG were assessed. In addition, the Inventory of Drug Taking Situations (IDTS) was evaluated. It is a 50 item self-reported questionnaire designed to assess the situational antecedents to the use of a wide range of drugs of abuse. The IDTS total score correlated with self-ratings of the severity of the patient’s substance use problem [20]. These analyses were carried out on patients with data assessed at both V1 and V4, except for the levomethadone exposure, which was assessed among all patients included in Part 1 of the study.
Statistical analysis
For quantitative variables, mean and standard deviations were calculated, while for qualitative variables the absolute and relative frequency distributions were used.
Results
Study population
A total of 88 patients were screened and enrolled in the study at the date of cut-off. Of these, 53 (60%) were included in this interim analysis; 47 patients (89%) completed Part 1 of the study and six (11%) abandoned the study before V4: 2 of these passed directly to Part 2 and four dropped out (two for loss of FU and two due to the patient’s decisions). Consequently, 49 out of 53 patients (92%) continued maintenance treatment with any drug in Part 2 of the study (Figure 2).
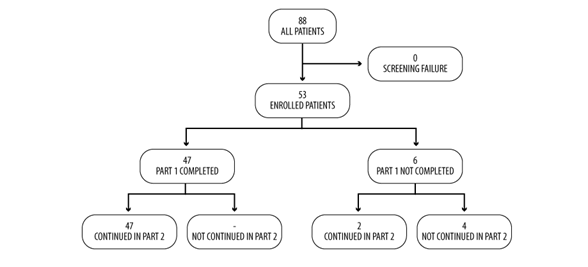
Figure 2: Flowchart of patientâ??s disposition in Part 1 of the study.
Baseline characteristics of patients
Baseline data of patients included in the interim analysis are presented in Table 1.
| Variable | N (%)/mean ± SD |
|---|---|
| Male Female |
47 (88) 6 (12) |
| Age (years) Frequency: 18â??30 years 31â??40 years 41â??50 years 51â??60 years >60 years |
42 ± 9 6 (11) 13 (25) 24 (45) 9 (17) 1 (2) |
| Bodyweight (kg) | 83 ± 17 |
| Height (cm) | 175 ± 6 |
| BMI (kg/m2) | 27 ± 4 |
| Hepatobiliary disorders Chronic hepatitis Hepatic cirrhosis |
2 (4) 1 (2) 1 (2) |
| Infections and infestations: Chronic hepatitis C Endocarditis HIV infection Hepatitis C Intervertebral discitis |
9 (17) 1 (2) 1 (2) 5 (9) 6 (11) 1 (2) |
| Investigations: Prolonged QT interval (ECG) |
1 (2) 1 (2) |
| Metabolism and nutrition disorders: Diabetes mellitus Dyslipidaemia Obesity Type 2 diabetes mellitus |
6 (11) 2 (4) 1 (2) 3 (6) 2 (4) |
| Musculoskeletal and connective tissue disorders: Intervertebral disc protrusion Polyarthritis |
2 (4) 1 (2) 1 (2) |
| Neoplasms (any type): Non-Hodgkin's lymphoma |
1 (2) 1 (2) |
| Nervous system disorders: Cervical myelopathy Epilepsy Extrapyramidal disorder |
3 (6) 1 (2) 1 (2) 1 (2) |
| Psychological/psychiatric disorders: Alcoholism Anxiety Anxiety disorder Attention deficit/hyperactivity disorder Borderline personality disorder Depression Dysphemia Insomnia Psychotic disorder Personality disorder (others) |
10 (19) 1 (2) 3 (6) 2 (4) 1 (2) 1 (2) 4 (8) 1 (2) 1 (2) 1 (2) 1 (2) |
| Respiratory, thoracic and mediastinal disorders: Asthma Sleep apnea syndrome |
2 (4) 1 (2) 1 (2) |
| Surgical and medical procedures: Spleen operation Tricuspid valve replacement |
1 (2) 1 (2) 1 (2) |
| Vascular disorders: Hypertension |
5 (10) 5 (10) |
Table 1: Baseline characteristics (n=53).
At the baseline visit, clinically significant abnormalities (as per the clinician’s assessment) were found in nine patients (17%); the most frequent were cardiovascular alterations and abnormalities in the abdominal body areas (5 patients each, 9%). Of these, two were pre-existing and related to drug assumption (hypertension and one abnormal parameter as inferior hepatic edge). Abnormalities in vital signs were reported by one patient (2%) and were not related to any drug.
Concomitant medications different from maintenance treatment at V1 were mainly psycholeptics (nine patients, 17%).
With regards to serology tests, they were performed in 50 out of 53 patients. in In total, 43 patients (86%) were HIV negative, 31 patients (62%) were HCV negative and 44 patients (88%) were HBV negative.
Previous maintenance treatments
Heroin was the most frequently abused drug (37 patients, 70%).
At V1, the mean duration of use of levomethadone was 317.3 days (SD=236.6).
Before switching to levomethadone maintenance treatment, 42 patients (79%) were treated with racemic methadone, whereas no previous maintenance opioid treatment was reported for the other patients.
The dose of racemic methadone at the switch to levomethadone ranged from 20 to 420 mg (mean ± SD=105.4 ± 61.5 mg).
Regarding the tolerability of previous treatment, 22 patients (41%) had at least one problem with racemic methadone and two had problems either with racemic methadone or buprenorphine/naloxone.
Patient’s concerns with racemic methadone were mainly referred to a lack of efficacy (10 patients, 45%) and other reasons (five patients, 22%) as weight gain (three patients, 6%), obesity (one patient, 2%) and QTC prolongation (one patient, 2%).
Safety analysis
Initial ADR: In total, seven ADRs (six non-serious and one serious) were registered in this interim analysis, experienced by four out of 53 patients (8%). About the suspected drugs, five ADRs were related to levomethadone (2 patients), one to Peg-IFN and one to benzodiazepine.
The most frequent ADRs were related to the category of psychiatric disorders, including those caused by levomethadone.
The most frequent ADR leading to drug discontinuation were also related to psychiatric disorders: five events in four patients (6%) regardless of the related drug and four events in two patients (4%) related to levomethadone.
Concerning the serious ADR, one patient experienced diabetes mellitus related to Peg IFN use.
QTc prolongation
QTc changes were evaluated in 21 patients for which data are available at both V1 and V4. Mean ± SD QTc values remained stable (429.3 ± 26.9 ms at V1, 427.1 ± 21.8 ms at V4), with a non-clinically significant mean difference between visits equal to -2.2 ms (SD=18.2). Details about QTc values according to levomethadone dose for each patient are represented in Figure 3.
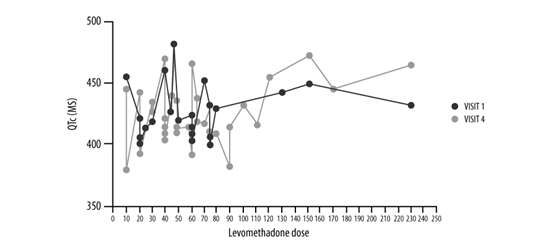
Figure 3: QTc values according to levomethadone dose (mg) per patient, evaluated at V1 (blue line) and V4 (red line) (n=21).
Of note, QTc variations were observed in both directions, as a decrement of QTc values between 30 and 59 ms was registered in two patients (10%), and between 1 and 29 ms in nine patients (43%); on the contrary, there was an increment between 1 and 29 ms in nine patients (43%) and between 30 and 59 ms in one patient (5%).
Considering the distribution of QTc values with respect to the specific threshold of 450 ms [21], this was: <450 ms in 31 out of 37 patients (84%) at V1 and in 23 out of 27 patients at V4 (85%); between 451 and 480 ms in six patients (16%) at V1 and in 3 (11%) at V4; between 481 and 500 ms in one patient (4%) at V4.
Overall, one patient only showed a clinically significant QTC prolongation that, however, remained below the threshold of risk as defined by international guidelines on pharmacovigilance (see FDA guidelines).
Evaluation of laboratory examinations
Laboratory examinations abnormalities were evaluated in at least 24 patients for each parameter, with a maximum of 38.
With respect to hematological evaluations, clinically significant abnormalities (as per the clinician’s assessment) were reported for six patients, either at V1 or at V4 (Table 2).
| n (%) | |
|---|---|
| ADR regardless of the related drug | |
| At least one ADR At least one serious ADR At least one ADR leading to discontinuation* |
4 (8) 1 (2) 4 (8) |
| ADR related to levomethadone | |
| At least one ADR At least one serious ADR At least one ADR leading to discontinuation*§ |
2 (4) - 2 (4) |
| Intensity category of ADR regardless of the related drug | |
| At least one mild ADR At least one moderate ADR At least one severe ADR |
1 (2) 4 (8) 1 (2) |
| Intensity category of ADR related to levomethadone | |
| At least one mild ADR At least one moderate ADR At least one severe ADR |
1 (2) 2 (8) â?? |
| *Leading to discontinuation of the related drug. §These 2 patients did not complete Phase 1 and went directly to the Phase 2 of the study. |
|
Table 2: Number of patients with at least one initial ADR.
Twelve patients reported at least one biochemical parameter outside the normal range at V1 and V4 (Table 3).
Parameter |
Patients with clinically significant abnoralities, n (%) |
|---|---|
Hematology:
|
2 (5) out of 38 evaluated |
Biochemistry:
|
3 (8) out of 37 evaluated |
Table 3: Distribution of abnormalities in laboratory examinations at V1 or V4.
Concerning the urine analysis, most of the values remained unchanged from V1 to V4. To be noted, leucocytes values decreased in 18% of patients (n=6) and increased in 24% of patients (n=8). There was also an increased presence of blood or urobilinogen in urine among 21% of patients (n=7) for both parameters.
Secondary aims
Effectiveness: Addiction Severity Index
• No significant changes at V4 with respect to V1 were evidenced for all composite scores, except for the psychic status (SD 0.1; 95% CI: -0.06 to -0.01).
Craving
• There was a significant decrease of VAS mean values related to the evaluation of craving from 17.5 (SD=18.2) at V1 to 8.3 (SD=15.9) at V4 (p=0.01). The overall mean change was equal to -9.2 points (SD=22.1; 95% CI: -15.8–2.7). Detailed VAS scores are summarized in Table 4.
| Time | n | VAS score (mean ± SD) |
|---|---|---|
| V1 | 46 | 17.5 ± 18.2 |
| V2 | 46 | 11.8 ± 14.5 |
| V3 | 47 | 11.5 ± 17.6 |
| V4 | 46 | 8.3 ± 15.9 |
Table 4: Mean VAS scores related to craving.
Quality of life
Quality of life evaluation was possible for 46 patients.
Concerning the Physical Component Summary, there was a little increase of values between V1 and V4 (from 48.6 ± 9.6 to 50.0 ± 8.0) with an overall mean change equal to 1.4 ± 8.3.
Similar results were obtained for the Mental Component Summary, with a non-significant mean increase equal to 2.8 ± 10.0 between V4 and V1 (from 44.6 ± 11.3 to 47.4 ± 10.7).
Catabolites
The evaluation of urine tests for catabolites of abused drugs was carried out on 47 patients.
Few patients executed a urine test between the IC signature and V1 (three patients, 6.4%) whereas a urine test was executed by 26 patients (55%) at V1, by 13 patients (28%) at V2, by 19 patients (40%) at V3, and by 14 patients (30%) at V4.
Catabolites were found in few patients for heroin, buprenorphine and cocaine (no more than 17%) whereas, with regards to methadone, the test resulted in positive in almost 90% of patients during the study period.
As regards the change of test results between the first and the last month in the study, for few patients the result changed for heroin, buprenorphine and cocaine whereas with methadone, a change from positive to negative was found in24 out of 37 patients (65%).
History of substance use (Patient’s diary)
The evaluation of diary information was performed on 45 patients. A total of 12 patients (26%) reported the use of a substance from V1 to V2 (1 month period), 14 patients (31%) from V2 to V3 (2 month period) and 11 patients (24%) from V3 to V4 (3 month period). A total of 28 patients (62%) reported no history of use of substances during Part 1 of the study. Details about substance consumption stratified for the study period are summarized in Table 5. No use of buprenorphine was reported.
| Period | Use of heroin, number of patients (%) | Use of cocaine, number of patients (%) |
|---|---|---|
| V1â??V2 | 8 (18) | 8 (18) |
| V2â??V3 | 8 (18) | 11 (24) |
| V3â??V4 | 6 (13) | 9 (20) |
Table 5: Substances using during the study period.
Retention in treatment
The statistic evaluation of the retention rate (the proportion of patients assuming Levomethadone during the study) using a Kaplan Meyer survival test was not carried out because the event to be studied, “stop of Levomethadone treatment during Phase I of the study”, occurred in too few patients.
The mean duration of levomethadone assumption was 494 days (SD=250), ranging from 55 to 967 days (median 528 days). A total of 49 out of 53 patients (92%) assumed levomethadone for more than 6 months.
Other measures
Levomethadone exposure: Overall, the levomethadone mean exposure was 65.7 mg (ranging from 6.5 to 235 mg, median=60 mg).
The mean administered dose of levomethadone was 65.2 mg (range: 7.0–235 mg, median=60 mg) at V1 and 64.7 (6.0–235 mg, median=60 mg) at V4.
In the 55% (n=29) of patients, levomethadone was administered with a mean dose ≥60 mg, in a percentage equal to 15% (n=8) it was administered with a mean dose between 50 and 59 mg and in a percentage equal to 30% (n=16) it was administered with a mean dose <50 mg.
Levomethadone blood levels: The mean blood level of levomethadone was 270 μg/L at V1 and 259 μg/L at V4. A good correlation (Pearson’s Correlation Coefficient=0.79) was found between blood level and dose administered of levomethadone at V3.
Abnormalities in physical examinations, vital signs and ECG
Six patients out of 46 (13%) reported at least one clinically significant abnormality at V1 or V4 physical examinations. The most frequent were in the abdominal body area.
Considering vital signs, one patient at V2 and 2 patients at V4 were judged to have a clinically significant abnormality.
Regarding ECG results, two patients out of 21 (9%) had QRS interval values at V4 >100 ms, with an increment from V1 >25%.
Inventory of drug taking situations
Drug Taking Situation (IDTS) analysis was carried out on 40 patients. Drugs assessed were heroin (30 patients, 75%), opioid (four patients, 10%), cocaine (three patients, 7%), methadone (one patient, 2%), morphine (one patient, 2%) and psychotropic drugs (one patient, 2%).
By considering the changes of V4 from V1, no significant changes were found in the mean values of scores for drug use during unpleasant emotions (-6.5; SD=25.0), physical discomfort (-3.8; SD=16.8), conflict with others (-5.5; SD=21.8); a significant decrease of mean scores between V4 and V1 were found for pleasant emotions (-7.8; SD=23.6), testing personal control (-10.3; SD=22.6), urges and temptations (-10.5; SD=26.1), social pressure (-12.0; SD=30.4) and pleasant time with other (-10.0; SD=25.3). No assumptions of second drugs were reported.
Discussion
Up to date, in the European context, only limited data are available regarding the efficacy and safety of long term treatment with levomethadone.
The population included in this interim analysis was composed of 53 patients in maintenance treatment with levomethadone. Among them, 47 patients (89%) completed Part 1 of the study.
Only non-serious ADRs related to levomethadone were reported by two patients, mainly classified as psychiatric disorders. Nevertheless, in these patients, the ADRs led to levomethadone discontinuation. The knowledge and proper management of the events that led to treatment discontinuation, regardless of their severity, could be useful to improve treatment adherence and would deserve a warning before starting the treatment.
As per arrhythmic risk, the present preliminary report documents a very safe profile of levomethadone. None of the study patients experienced a QTc prolongation beyond the risk thresholds, namely an absolute value >480 ms, or a prolongation greater than 60 ms. Also, no events suggestive for tachyarrhythmias risk were reported during this FU.
With respect to hematology, few values were found abnormal, while 12 patients reported at least one biochemistry parameter outside the normal range. These involve fasting glucose, AST, ALT, GGT, albumin, cholesterol, uric acid, and potassium parameters.
Taken together, these data support the safety profile of levomethadone therapy.
Within this interim analysis, levomethadone treatment produced a positive effect on craving, which showed a significant decrease between V1 and V4 (p=0.01). By the Addiction Severity Index score evaluation, a significant change for psychic status was also reported. The most of patients (62%) reported no substance use during Part 1 of the study. In addition, the IDTS highlights a significant decrease in drug use related to pleasant emotions, testing personal control, urges and temptations, social pressure and a pleasant time with others.
For instance, the urine test resulted positive for methadone catabolites in almost 90% of patients during the study period. This finding is of difficult interpretation. The urine examination was carried out according to the routine clinical practice of the participant centers and not by a centralized procedure. Therefore, this result could be attributed to the non-discrimination between racemic methadone and levomethadone.
Only two previous works allowed an evaluation of the retention in treatment with levomethadone used as a long term treatment. In detail, Riglietta and collaborators observed retention in treatment of 93% of patients at 6 months and the paper by
Consoli reported a mean time of retention to treatment equal to 15.3 ± 8.0 months [9,22]. Even if it was not possible a statistical evaluation of the retention in treatment in this interim analysis, we observed that most patients (92%) assumed levomethadone for more than 6 months.
Of note, the levomethadone mean exposure was 65.7 mg and corresponds to about half of the mean ± SD dose of racemic methadone before the switch to levomethadone, which was equal to 105.4 ± 61.5 mg.
Conclusion
Results from this interim analysis show that a long term levomethadone therapy, administered to patients with opioid addiction, is effective in the treatment of disease, with a reduction in craving and opioid use.
The data relating to safety and, in particular, to the possible correlation with the length of the ECG QTc section, show a favorable risk/benefit ratio.
In conclusion, even if these results are preliminary and should be integrated with the final results of both Part 1 and 2 of the study, data collected in the present interim analysis are very encouraging and support the good effectiveness and safety profile of levomethadone therapy.
Acknowledgements
Editorial assistance was provided by Simonetta Papa, PhD, and Aashni Shah (Polistudium SRL, Milan, Italy). Graphical assistance was provided by Massimiliano Pianta (Polistudium SRL, Milan, Italy). This assistance was supported by L. Molteni and C.
The Authors would like to thank all the principal investigators of the participant centers: Dr. Giovanni Strepparola, UOC Servizio Territoriale delle Dipendenze Sede di Gorgozola, Milan; Dr. Franco Montesano, Struttura Semplice Ser. D Soverato ASP, CZ, Soverato; Dr. Pietro Casella, UOS Dipendenze, ASL Roma 1, Roma; Dr. Filomena Romano, UOC Ser. D – ASL Avellino, Avellino; Dr. Luigi Andreozzi, U.O.C. Ser. D Aversa Dipartimento Dipendenze – ASL Caserta, Aversa; Dr. Maria Nunziata Varvara, Ser. D. Altamura UOC del Sud Barese Dipartimento di Dipendenze Patologiche – ASL Bari, Altamura; Dr. Roberta Ballestra, Azienda Sanitaria Universitaria Giuliano Isontina (ASUGI), Struttura Complessa Dipendenza da Sostanze Illegali – Dipartimento delle Dipendenze, Trieste; Dr. Carrozzino Roberto, S.C. Ser.T. – Dipartimento Salute Mentale e Dipendenze – ASL 2, Savona.
Funding
This study was sponsored by L. Molteni and C.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Availability Of Data And Material
Data may be made available upon reasonable request.
Ethics Approval
The ethics committee of all participant centers reviewed and approved the study protocol.
Consent to Participate
All the participants signed an informed consent form.
Consent for Publication
Not required as this manuscript does not include details, images or videos related to the participants.
Authors Contributions
Study design: AC; data collection and interpretation: All; manuscript writing: All; manuscript editing and approval to submit: All.
References
- M. J. Krantz, P. S. Mehler, Treating opioid dependence. Growing implications for primary care, Arch Intern Med,164(2004),277-88.
- M. A. Schuckit, Treatment of opioid-use disorders, N Engl J Med,375(2016),357-68.
- N. Sanger, M. Bhatt, L. Zielinski, S. Sanger, H. Shahid, et al., Treatment outcomes in patients with opioid use disorder initiated by prescription: A systematic review protocol. Syst Rev, 7(2018),16.
- European Drug Report, COVID-19, Drug use and harms, Drug markets 2020.
- C. E. Inturrisi, Pharmacology of methadone and its isomers. Minerva Anestesiol, 71(2005),435-7.
- M. Meini, M. Moncini, L. Daini, D. Scramelli, M. Miliantie, et al. Opioid dependence treatment: Is levomethadone a new frontier? A pilot study in Italy,J Toxic Pharm,1(2017),012.
- S. B. Karch, Is it time to reformulate racemic methadone?, J Addict Med,5(2011),229-31.
- K. Kristensen, T. Blemmer, H. R. Angelo, L. L. Christrup, N. E Drenck, et al., Stereoselective pharmacokinetics of methadone in chronic pain patients. Ther Drug Monit, 18(1996),221-7.
- M. Riglietta, P. Donadoni, G. Carbone, C. Pisoni, G. Plebani, et al., Lâ??esperienza clinica con Levometadone nel trattamento del disturbo da uso di oppiacei, Mission, 52(2019), set.
- N. Ansermot, O. Albayrak, J. Schläpfer, S. Crettol, M. Croquette-Krokar, et al. Substitution of (R,S)-methadone by (R)-methadone: Impact on QTc interval, Arch Intern Med, 170(2010),529-36.
- P. P. Pani, E. Trogu, I. Maremmani, M. Pacini, QTc interval screening for cardiac risk in methadone treatment of opioid dependence, Cochrane Database Syst Rev,6(2013),CD008939.
- J. Lötsch, C. Skarke, J. Wieting, B. G. Oertel, H. Schmidt, et al., Modulation of the central nervous effects of levomethadone by genetic polymorphisms potentially affecting its metabolism, distribution, and drug action, Clin Pharmacol Ther,79(2006),72-89.
- A. Vendramin, A. M. Sciacchitano, Pharmacology and neurochemistry of methadone. Heroin Addict Relat Clin Probl, 11(2009),11-28.
- A. Ferrari, C. P. Coccia, A. Bertolini, E. Sternieri. Methadone metabolism, pharmacokinetics and interactions, Pharmacol Res, 50(2004),551-9.
- A. Giovanni,Summary of Product Characteristics, SmPCâ?? Ellepalmiron, Methadone hydrochloride Molteni,2020.
- Addiction Severity Index. Centro Scientifico Editore, II edizione, 2001.
- A. Consoli, A. Bernardo, Diagnosi e valutazione nelle tossicodipendenze e nellâ??alcolismo. Addiction severity index. 2001 â?? Centro scientifico editore. ISBN: 8876405623
- C. M. Denis, J. S. Cacciola, A. I. Alterman, Addiction Severity Index (ASI) summary scores: comparison of the Recent Status Scores of the ASI-6 and the Composite Scores of the ASI-5, J Subst Abuse Treat, 45(2013):444-50.
- Apolone. Questionario sullo stato di salute SF-12 Versione italiana. Istituto di Ricerche Farmacologiche Mario Negri. Milano 2005. SF-12 health questionnaire Italian version.
- N. E. Turner, H. M. Annis, S. M. Sklar, Measurement of antecedents to drug and alcohol use: psychometric properties of the Inventory of Drug-Taking Situations (IDTS). Behav Res Ther,35(1997),465-83.
- Health Canada - Guide for the Analysis and Review of QT/QTc Interval Data,2020.
- A. Consoli, Effectiveness, tolerability and safety of a long-term therapy with levomethadone in clinical practice: A retrospective observational study. Heroin Addiction and Related Clinical Problems 2020 Published Ahead of Print, September 17, 2020.

