Research Article: Journal of Drug and Alcohol Research (2025) Volume 14, Issue 10
Effects of Methoxyflavone and their Derivatives on Memory Deficits Induced by Scopolamine in Zebrafish Models of Alzheimer’s Disease
Nithya Panneerselvam1*, Parimala Kathirvelu2 and Hemanthkumar Shanmugam32Department of Pharmacology, Meenakshi Medical College Hospital and Research Institute, Meenakshi Academy of Higher Education and Research, Deemed to be University, Kanchipuram, T, India
3Department of Pharmacology, Sri Venkateswaraa Medical College and Research Institute, Chennai, Tamilnadu, India
Nithya Panneerselvam, Department of Pharmacology, Faculty of Medicine, Sri Lalithambigai Medical College and Hospital, Dr. MGR Educational and Research Institute, Chennai, Tamilnadu, India, Ph.D Schol, India, Email: nithyapanneerselvam83@gmail.com
Received: 21-Oct-2025, Manuscript No. JDAR-26-176236; Editor assigned: 28-Oct-2025, Pre QC No. JDAR-26-176236 (PQ); Reviewed: 22-Dec-2025, QC No. JDAR-26-176236; Revised: 24-Dec-2025, Manuscript No. JDAR-26-176236 (R); Published: 31-Dec-2025, DOI: 10.4303/JDAR/236475
Abstract
Background: Dementia, predominantly caused by Alzheimer’s disease (60-70% of cases), represents a significant disease burden. As methoxyflavones from Kaempferia parviflora possess AChE-inhibition properties, synthetic methoxyflavones and their derivatives were chosen for their therapeutic potential in established zebrafish neurodegenerative models.
Aim: To study the effect of methoxyflavone and its synthetic derivatives in scopolamine-induced memory deficit models of Zebrafish.
Methodology: 12 groups of adult Danio rerio (10 fish per tank) were used. A stock solution of all three test drugs (7-methoxyflavone, 7,4’ dimethoxyflavone, and 7,2’,4’ trimethoxyflavone-in doses of 5,10,20 mg), standard Rivastigmine 1.5 mg/kg, and scopolamine 200 μM were prepared & diluted to the desired volume of stock solution in 1 L of system water. Each animal in the designated group was exposed to the drug containing fluid for 60 minutes. Then they were subjected to behavioural models of learning and memory.
Results: In the T-maze analysis, 7-methoxyflavone 20 mg/L showed a more extended time spent ratio (1.230 ± 0.207) and a greater total number of entries ratio (1.4261 ± 0.352) in the green arm, which were moderately comparable with the time spent ratio (1.9568 ± 0.228) and the number of entries ratio (2.345 ± 1.013) in the Rivastigmine group. In the light and dark test, zebrafish that received 7-methoxyflavone at doses of 5 mg/L (151 ± 6.548), 10 mg/L (169.1 ± 7.950), and 20 mg/L (176.2 ± 6.442) had significantly increased the time spent in the light chamber in comparison to the Rivastigmine group (220.9 ± 6.154).
Conclusion: 7-Methoxyflavone in a dose of 20 mg/L exhibited significant cognitive improvement in scopolamine-induced memory deficit models of Zebrafish. These results position it as a prime candidate for further development and research in the treatment of Alzheimer’s disease dementia.
Keywords
Methoxyflavone; Zebrafish; Dementia; Alzheimer’s disease; Light and dark test; T-maze test
Introduction
Neurodegenerative diseases are progressive neurological disorders characterized by the loss of neurons that is irreversible, causing profound impairments in cognition, movement, and behavior. Dementia is the leading disease burden; about 60-70% of all dementia cases are due to Alzheimer’s disease [1]. Dysfunction within the cholinergic system is widely recognized as an important factor in the early stage of AD development, as supported by various findings of disrupted cholinergic functions in both patients with AD and animal models thereof. One of the early pathophysiological events common to AD is the deficit of acetylcholine, also known as ACh, which is a neurotransmitter important for memory and learning. The need for such a deficit is primarily the enzymatic destruction of ACh via acetylcholinesterase, AChE, and to a lesser degree, butyrylcholinesterase [2-4]. Considering the involvement of cholinesterase enzymes in the pathology of AD, inhibitors of cholinesterases became the first therapeutic strategy developed against the disease. However, despite the detection of other molecular targets for AD, cholinesterase inhibitors are still the most prescribed drugs for the treatment of mild to moderate stages of the disease. They act by promoting synaptic plasticity and enhancing acetylcholine availability. Further to cholinergic dysfunction, the accumulation of amyloid beta plaques and the presence of abnormal tau protein are among other important factors in AD progression [5,6].
Although several animal models exist that are used for screening neurodegenerative disorders, many of these models only partially emulate the pathology of AD. zebrafish, Danio rerio, have recently become an increasingly encouraging model in the study of such diseases, offering a wider range of molecular and behavioral features related to Alzheimer’s disease.
Zebrafish genes are 70% homologous with human genes; 84% of human disease-causing genes are reported to have their zebrafish counterparts, which underscores their value and relevance as translational models. The general structure of the zebrafish brain is significantly conserved compared to that of mammals. Both the zebrafish and mammalian brains are similarly organized into the hindbrain, midbrain, and forebrain, further divided into the telencephalon, mesencephalon, metencephalon, and myelencephalon. Many specific areas, such as the olfactory bulbs, hypothalamus, and cerebellum, are also similarly defined within these regions [7-9]. The BBB of a zebrafish also structurally and functionally resembles the mammalian BBB, making it useful for permeability studies of new neurodrugs [10].
Moreover, the cognitive abilities of zebrafish can be evaluated through several paradigms for testing their learning and memory, many of which parallel those used in rodent studies [11-13].
Besides that, Kaempferia parviflora has demonstrated promising potential in cholinesterase inhibition. In the case of compounds isolated from the plant, 5,7,4’- trimethoxyflavone and 5,7-dimethoxyflavone have demonstrated the most potent inhibiting activity against AChE and BChE enzymes, whose inhibition activity has been reported to lie in the range of 43–85% according to Pattara Sawasdee, et al. [14]. In addition, a structureactivity relationship identified methoxy groups at the 5, 7, and 4’ position as important for AChE inhibition. As only limited studies exist concerning AD, this study evaluates the impact of different doses of three related synthetic compounds 7-methoxyflavone, 7,4’-dimethoxyflavone, and 7,2’,4’-trimethoxyflavone on the cognitive functions of zebrafish through behavioural tests like T-maze and lightand- dark chamber tests.
Materials and Methods
An eight-month-old male adult Danio rerio will be used in this neurocognitive function study.
The zebrafish will be kept in a 10-liter tank with an aerator to maintain enough oxygen. The tank’s ambient temperature will be maintained at 25 ± 2°C, with a photoperiod of 11 to 12 hours of light and 11 to 12 hours of dark.
The adult male zebrafish were divided into 12 groups by average body weight in such a manner that each group consisted of 10 fish, which were housed in a 2-liter circulating tank. The body weight for the fish in each group ranged from 0.31-0.41 g. Before starting the actual experiment, the zebrafish would be acclimatized for two weeks to get used to the conditions of housing. All fish were acclimatized to the treatment chambers before the test sessions.
A stock solution of all the three test drugs, 7- methoxyflavone, 7,4’-dimethoxyflavone, and 7,2’,4’-trimethoxyflavone, standard Rivastigmine, and Scopolamine, was prepared. From the stock solution, drugs at the desired concentration were prepared by dilution of a desired volume of stock solution in 1 L of system water. Standard drug 1.5 mg/L Rivastigmine and Scopolamine (200 μM) were dissolved in water, while in the control group, system water from the maintenance tank was used.
The controlled behavioral observations will take place between 9:30 AM and 1:00 PM to minimize neuroendocrine fluctuations that may interfere with neurobiological or neurobehavioral outcomes.
Experimental study groups:
Group I: Control (fed by water)
Group II: Scopolamine 200 μM (SCO)
Group III: Scopolamine 200 μM+Rivastigmine 1.5 mg/ kg/L (SCO+RV)
Group IV: Scopolamine 200 μM+7,2’,4’ -trimethoxyflavone 5 mg/L (SCO+TMF-5)
Group V: Scopolamine 200 μM+7,2’,4’-trimethoxyflavone 10 mg/L (SCO+TMF-10)
Group VI: Scopolamine 200 μM +7,2’,4’ -trimethoxyflavone 20 mg/L (SCO+TMF-20)
Group VII: Scopolamine 200 μM+7,4’-dimethoxyflavone 5 mg/L (SCO+DMF-5)
Group VIII: Scopolamine 200 μM+7,4’ -dimethoxyflavone 10 mg/L (SCO+DMF-10)
Group IX: Scopolamine 200 μM+7,4’-dimethoxyflavone 20 mg/L (SCO+DMF-20)
Group X: Scopolamine 200 μM+7-methoxyflavone 5 mg/L (SCO+MF-5)
Group XI: Scopolamine 200 μM+7-methoxyflavone 10 mg/L(SCO+MF-10)
Group XII: Scopolamine 200 μM+7-methoxyflavone 20 mg/L(SCO+MF-20)
Experimental procedures
Colour-biased appetite conditioning T-maze test [15] zebrafish cognitive capacity was assessed using a T-maze test with color-biased hunger conditioning. The acrylic glass T-maze has one long arm (50 cm × 10 cm × 10 cm) and two short arms (20 cm × 10 cm × 10 cm). The zebrafish were given the test drug and Rivastigmine at a dosage of 1.5 mg/L two hours before the experiment. An hour prior to the experiment, a 200 μM scopolamine solution was administered. Every solution, including test extracts and standard drugs, was made fresh on the day of the experiment. Zebrafish was placed at the end of the long arm during the training. The sliding door opened after a minute, allowing the fish to swim. The sliding door at the intersection closes as soon as a fish enters the short arms. Fish in the short arms are watched for four minutes. The fish was rewarded with food if it had entered the green arm. However, if it entered the red arm, the training was repeated for three days in a row without any sustenance. Fish were subjected to the identical methods during the testing sessions, but they were not given food in the green arm or punishment in the red arm. Behavioral metrics of all fish were recorded for further analysis by measuring the time spent in each arm and total number of entries into both the red and green arms.
Light and dark preference test
The light and dark preference test has been a standard model for analysing cognitive and memory functions in zebrafish. The experimental apparatus consisted of a non-reflective rectangular acrylic tank (15 ×10 × 45 cm) that was designed to avoid shoaling behaviour based on reflection. The tank had sliding central doors with the same color as the tank sides. The water level in the tank was kept at a height of 10 cm. Illumination was provided by a 500–600 lux bulb placed 1.8 m above the tank, and trials were recorded with a video camera positioned 50 cm above the setup [16].
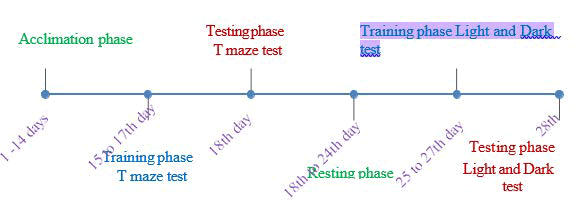
Each fish was acclimatized for two minutes in the middle chamber of the tank before the session. Following the acclimatization phase, the sliding door was opened, and the fish allowed to explore both the light and dark chambers for 15 minutes. Throughout the training sessions, rewards in the form of food were given upon entry into the light chamber, while entry into the dark chamber was disturbed by a glass rod. Fish selecting the dark chamber continuously were retrained for three consecutive days. There was no food reward in the light chamber, nor any disturbance in the dark chamber during each of the testing sessions. Behavioural parameters-time spent and number of entries in both the light and dark chambers were recorded and analyzed to study learning and memory performance (Figure 1).
Figure 1: Experimental design of this study
Statistical analysis
Sigma Plot (version 12.0) was used to analyse the data. The mean ± SEM was used to express all values. The Mann Whitney U test was used for multiple comparisons, and the Kruskal-Wallis test was used for data analysis. At p<0.05, differences between the groups were deemed statistically significant.
Results
Colour-biased appetite conditioning T-maze test
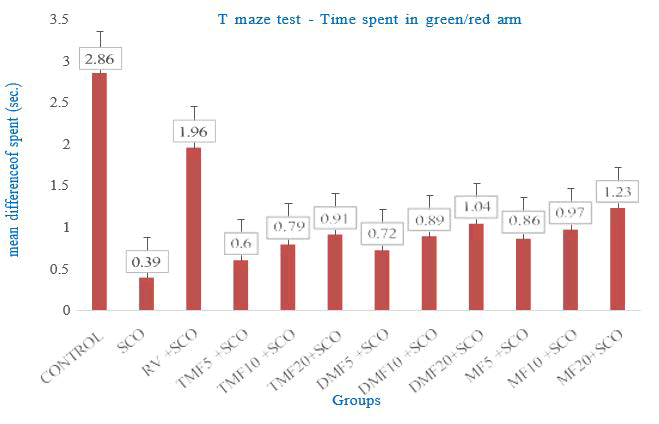
As seen in Figures 2 and 3, the time spent and the total number of entries in the green/red arms were recorded, and the mean difference (green/red arm) was computed. It was determined that the zebrafishes spent more time in the green arms than in the red arms for groups showing a mean difference larger than 1 in both time spent and total number of entries. This shows that the zebrafish in these groups had high memory. Rivastigmine and test medications (7,4’-dimethoxy flavone 20 mg/L and 7-methoxyflavone at all doses) caused zebrafish to exhibit a mean difference in time spent and total number of entries greater than 1. Zebrafish that received 7-methoxyflavone showed a more extended time spent ratio and a greater total number of entries ratio, which were moderately comparable to those of the positive control (Figure 2).
Figure 2: Effect of Scopalamine (SCO), Rivastigmine (RV), TMF (7,2’,4’-trimethoxyflavone), DMF (7,4’-dimethoxyflavone), MF (7-methoxyflavone) on time spent in the colour-biased appetite conditioning T-maze test
The mean difference in time spent between the green and red arms was expressed as mean ± SEM. Zebrafish that received RV+SCO, DMF20+SCO, MF20+SCO showed mean difference of time spent at more than 1 as 1.95 ± 0.228, 1.041 ± 0.099 and 1.230 ± 0.207, respectively (Figure 3).
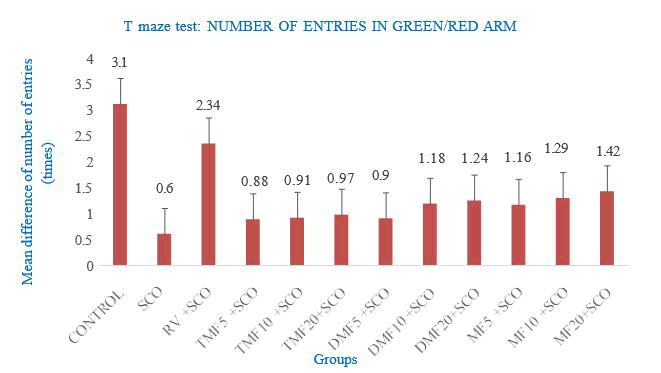
Figure 3: Effect of SCO, RV, TMF (7,2’,4’-trimethoxyflavone), DMF (7,4’-dimethoxyflavone), MF (7-methoxyflavone) on number of entries in the colour-biased appetite conditioning T-maze test
The mean difference in the total number of entries into the green and red arms was expressed as mean ± SEM. Zebrafish that received RV+SCO, DMF10+SCO, DMF20+SCO, MF5+SCO, MF10+SCO, MF20+SCO showed mean difference of total number of entries more than 1 as 2.34 ± 1.01, 1.18 ± 0.34, 1.24 ± 0.67,1.16 ± 0.33,1.29 ± 0.49 and 1.42 ± 0.35, respectively.
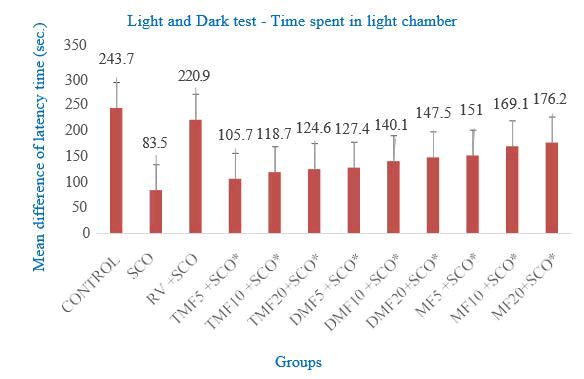
Zebrafish that received 7-methoxyflavone at doses of 5, 10, and 20 mg/L had significantly increased the time spent in the light chamber, i.e., increased latency time as 151 ± 6.548, 169.1 ± 7.95 and 176.2 ± 6.442 seconds, respectively, compared to the scopolamine group (83.5 ± 13.083) as shown in Figure 4.
Figure 4: Effect of SCO, RV, TMF (7,2’,4’-trimethoxy flavone), DMF (7,4’-dimethoxyflavone), MF (7-methoxyflavone) on the time spent in light chamber–light and dark test
7,2’,4’-trimethoxyflavone and 7,4’-dimethoxyflavone showed moderate results when compared to 7-methoxyflavone. The preference for the light chamber was considered an improvement in memory function. The control and rivastigmine groups showed more preference for the light chamber. The entries to the dark chamber were relatively more in 7,2’,4’-trimethoxyflavone treated zebrafish.
Discussion
This study investigates the potential of methoxyflavones and their derivatives to mitigate scopolamine-induced dementia in zebrafish, a widely used model for studying memory impairment. Scopolamine, a potent muscarinic receptor antagonist, disrupts cholinergic signalling by preventing acetylcholine from binding to its postsynaptic receptors. This is manifested by an increased activity of AChE, enhanced oxidative stress and neuroinflammation, and an increased expression of APP and Tau proteins, which are common hallmarks of neurodegeneration in AD [17].
The outcomes indicated that cognitive disturbances in zebrafish, such as passive avoidance and learning response impairment, are a reliable simulation of the symptoms produced in AD conditions. Rivastigmine, an already known acetylcholinesterase inhibitor and clinically used medication for AD, was used as a positive control to validate the ability and neurotransmitter ACh. The organ responsible for memory of this type, which involves learning, is the hippocampus. Against this background, destruction of the hippocampal cells leads to disturbances in memory functions and loss of short-term memory, which is reflected in the inability of animals to learn and perform appropriate behavioral patterns. In the T-maze test, four colors (blue, green, red, and yellow) were used to train the zebrafish.They showed a higher response to red and green than to the remaining colors. Consequently, these colors were used in the experiments, and feeding was done in the green arm to enhance learning and memory through a phenomenon called synesthesia. The T-maze test measured the time spent in the green and red arms to evaluate zebrafish learning and memory [18-20]. The study found that in the colour-biased appetite conditioning T-maze test, the time spent ratio and the total number of entries in the green/red arm ratio greater than 1 indicated good memory performance. Zebrafish treated with Rivastigmine exhibited the highest time spent ratio and total number of entries ratio, suggesting that Rivastigmine significantly improved spatial learning and recognition. This improvement is attributed to Rivastigmine’s inhibition of Acetylcholinesterase (AChE) activity [21]. In this study, 7-methoxyflavone doses (5, 10, 20 mg/L) were tested, and the time spent ratio and the total number of entries in the green arm were significantly higher than in the red arm and compared to the positive control. 7,4’-dimethoxyflavone in a dose of 20 mg/L indicated a cognitive improvement when compared to its other doses of 5 and 10 mg/L and 7,2’,4’-trimethoxyflavone improved memory performance compared to the negative control. The light-dark preference test is a widely used behavioral model for assessing cognitive and memory functions in zebrafish, particularly in response to pharmacological treatments. In this test, a preference for the light chamber signifies improved memory function. Both the control group and the group treated with Rivastigmine showed a marked preference for the light chamber, indicating enhanced memory. Conversely, zebrafish treated with 7,2’,4’-trimethoxyflavone and 7,4’-dimethoxyflavone exhibited a higher number of entries into the dark chamber, suggesting a lesser degree of memory improvement compared to the control and rivastigmine groups. In contrast, 7-methoxyflavone doses showed increased latency time and significant time spent in the light chamber, indicating increased memory function. Thus proving it to be a potential compound for improving cognition in Alzheimer’s disease. This study supports the findings of the survey by Mhalhel, et al. on the effects of flavonoids in neurodegenerative disorders such as Alzheimer’s disease [14,21].
Conclusion
The effects of 7-methoxyflavone, 7,4’-dimethoxyflavone and 7,2’,4’-trimethoxyflavone on memory deficit induced by scopolamine were investigated. Results proved that methoxyflavone and its derivatives showed significant improvement in memory, and 7-methoxyflavones in all doses (5, 10 and 20 mg/L) had significant cognitive improvement in comparison with Rivastigmine. 7-methoxyflavone has the potential to be developed as a health product to help alleviate memory impairment or treat Alzheimer’s disease
Study Limitations
This study was conducted as a preliminary investigation to identify behavioural parameters of methoxyflavone and its derivatives. Our findings can be helpful for further research on methoxyflavone in biochemical estimation of Acetylcholinesterase activity (AchE) and Glutathione S-transferase (GST) and histopathological detection of degenerative changes in brain tissues of zebrafish, to prove it as one of the promising compounds in the management of dementia in Alzheimer’s disease.
Ethical Approval
The study was approved by the Institutional Animal Ethics Committee, Registration No: 863/PO/Re/S/04/CPCSEA in meeting dated 18/12/2021. The authors have no conflict of interest regarding this study
Financial Disclosure
The authors declare that no funds, grants, or other support were received for study
Author Contributions
Conceptualisation, methodology, software, validation formal analysis: PK, NP; Investigation resources, data curation, writing-original draft,writing-review and editing visualisation: PK,NP, HKS; Supervision: PK.
References
- C. Qiu, M. Kivipelto, E. von Strauss, Epidemiology of Alzheimer's disease: Occurrence, determinants, and strategies toward intervention, Dialogues Clin Neurosci, 11(2009):111-128.
[Crossref] [Google Scholar] [PubMed]
- Alzheimer's Association, 2021 Alzheimer's disease facts and figures, Alzheimers Dement, 17(2021):327-406.
- G. DF, E. Nichols, J.D. Steinmetz, S.E. Vollset, K. Fukutaki, et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019, Lancet Public Health, 7(2022):e105-e125.
- H.T. Ferreira-Vieira, M.I. Guimaraes, R.F. Silva, M.F. Ribeiro, Alzheimer's disease: Targeting the cholinergic system, Curr Neuro pharma, 14(2016):101-115.
[Crossref] [Google Scholar] [PubMed]
- W. Thangnipon, N. Puangmalai, R. Soi-Ampornkul, N. Suwanna, P. Tuchinda, et al. Neuroprotection of N-benzylcinnamide on scopolamine-induced cholinergic dysfunction in human SH-SY5Y neuroblastoma cells, Neural Regen Res, 12(2017):1492-1498.
[Crossref] [Google Scholar] [PubMed]
- K.J. Thompson, A.B. Tobin, Crosstalk between the M1 muscarinic acetylcholine receptor and the endocannabinoid system: A relevance for Alzheimer’s disease? Cell Signal, 70(2020):109545.
[Crossref] [Google Scholar] [PubMed]
- M. Newman, G. Verdile, R.N. Martins, M. Lardelli, Zebrafish as a tool in Alzheimer's disease research, Biochim Biophys Acta, 1812(2011):346-352.
[Crossref] [Google Scholar] [PubMed]
- A.V. Kalueff, A.M. Stewart, R. Gerlai, Zebrafish as an emerging model for studying complex brain disorders, Trends Pharmacol Sci, 35(2014):63-75.
[Crossref] [Google Scholar] [PubMed]
- S. Santana, E.P. Rico, J.S. Burgos, Can zebrafish be used as animal model to study Alzheimer's disease?, American J Neuro Dis, 1(2012):32-48.
[Google Scholar] [PubMed]
- T. Ayodele, E. Rogaeva, J.T. Kurup, G. Beecham, C. Reitz, Early-onset Alzheimer's disease: What is missing in research?, Curr Neurol Neurosci Rep, 21(2021):4.
[Crossref] [Google Scholar] [PubMed]
- F.E. Williams, D. White, W.S. Messer, A simple spatial alternation task for assessing memory function in zebrafish, Behav Processes, 58(2002):125-132.
[Crossref] [Google Scholar] [PubMed]
- D. Eddins, D. Cerutti, P. Williams, E. Linney, E.D. Levin, Zebrafish provide a sensitive model of persisting neurobehavioral effects of developmental chlorpyrifos exposure: Comparison with nicotine and pilocarpine effects and relationship to dopamine deficits, Neurotoxicol Teratol, 32(2010):99-108.
[Crossref] [Google Scholar] [PubMed]
- E.D. Levin, D. Sledge, S. Roach, A. Petro, S. Donerly, Persistent behavioral impairment caused by embryonic methylphenidate exposure in zebrafish, Neurotoxicol Teratol, 33(2011):668-673.
[Crossref] [Google Scholar] [PubMed]
- P. Sawasdee, C. Sabphon, D. Sitthiwongwanit, U. Kokpol, Anticholinesterase activity of 7-methoxyflavones isolated from Kaempferia parviflora, Phytother Res, 23(2009):1792-1794.
[Crossref] [Google Scholar] [PubMed]
- K. Maddula, V.P. Kumar, J. Anusha, Assessment of aqueous extract of Ocimum sanctum leaves in memory enhancement and preventing memory impairment activities in zebrafish model, JBCP, 3(2017):85-192.
- L.D. Magno, A. Fontes, B.M. Gonçalves, A. Gouveia, Pharmacological study of the light/dark preference test in zebrafish (Danio rerio): Waterborne administration, Pharmacol Biochem Behav, 135(2015):169-176.
[Crossref] [Google Scholar] [PubMed]
- S. Saleem, R.R. Kannan, Zebrafish: An emerging real-time model system to study Alzheimer’s disease and neurospecific drug discovery, Cell Death Discov, 4(2018):45.
[Crossref] [Google Scholar] [PubMed]
- T. Muller, Rivastigmine in the treatment of patients with Alzheimer’s disease, Neuropsychiatr Dis Treat, 3(2007):211-218.
[Crossref] [Google Scholar] [PubMed]
- S. Perathoner, M.L. Cordero-Maldonado, A.D. Crawford, Potential of zebrafish as a model for exploring the role of the amygdala in emotional memory and motivational behavior, J Neurosci Res, 94(2016):445-462.
[Crossref] [Google Scholar] [PubMed]
- Y.H. Kim, K.S. Lee, A.R. Park, T.J. Min, Adding preferred color to a conventional reward method improves the memory of zebrafish in the T-maze behavior model, Anim Cells Syst, 21(2017):374-381.
- K. Mhalhel, M. Sicari, L. Pansera, J. Chen, M. Levanti, et al. Zebrafish: A model deciphering the impact of flavonoids on neurodegenerative disorders, Cells, 12(2023): 252.
[Crossref] [Google Scholar] [PubMed]
Copyright: © 2025 Nithya Panneerselvam, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.