Research Article: Journal of Drug and Alcohol Research (2025) Volume 14, Issue 8
Dercum’s Disease with Associated with Autonomic Manifestations, Novel Hypotheses on Pathophysiology of Neuritic Pain and Drug Therapy
Lourdes de Fatima Ibanez Valdes1, Sibi Joseph2 and Humberto Foyaca Sibat3*2Department of Neurology, Nelson Mandela Academic Hospital, South Africa
3Department of Neurology, Nelson Mandela Academic Hospital, Walter Sisulu University, South Africa
Humberto Foyaca Sibat, Department of Neurology, Nelson Mandela Academic Hospital, Walter Sisulu University, South Africa, Email: humbertofoyacasibat@gmail.com
Received: 21-Apr-2025, Manuscript No. JDAR-25-165178; Editor assigned: 25-Apr-2025, Pre QC No. JDAR-25-165178 (PQ); Reviewed: 09-May-2025, QC No. JDAR-25-165178; Revised: 05-Aug-2025, Manuscript No. JDAR-25-165178 (R); Published: 02-Sep-2025, DOI: 10.4303/JDAR/236462
Abstract
Introduction: Dercum’s Disease (DD), also named Adiposis Dolorosa (AD). It is a chronic debilitating condition with various clinical presentation types confirmed through imaging. It is a sporadic syndrome characterized by multiple, painful, subcutaneous adipose tissues commonly localized in the abdomen and extremities. We reported a new case presenting different neurological manifestations.
Methods: We searched the literature, following the guidelines outlined in the PRISMA statement. From January 2023 to January 2025, the authors searched the scientific databases, Scopus, Embassy, Medline, and PubMed Central using the following searches: “Dercum’s disease” OR “adiposis dolorosa” OR “Pathophysiology of neuritic pain” OR “neuritic pain in adiposis dolorosa” OR “therapy of Dercum’s disease”, OR “mTOR in neuritic pain”.
Results: After screening the full-text articles for relevance, 22 articles were included for final review. However, no article was selected when we searched for DD/NP/documented pathogenesis/respond to drug therapy.
Conclusions: Based on the systematic review of the medical literature we also released novel hypotheses on the pathogenesis of neuritic pain in patients presenting DD. Obviously further investigations will support or reject our proposals.
Keywords
Dercum’s disease; Adiposis dolorosa; Symptomatic swelling; Subcutaneous fat masses; Neuritic pain; Pathogenesis of Dercum’s disease; Drug therapy for adiposis dolorosa
Introduction
Adiposis Dolorosa (AD) is another term for Dercum’s Disease (DD). Imaging demonstrates that it is a chronic, debilitating disease with a range of clinical manifestations. Numerous painful subcutaneous adipose tissues, typically found in the abdomen and extremities, are indicative of this sporadic disorder. The majority of writers believe that DD is a confluence of neurological and endocrine conditions. The formation of many painful lipomas inside subcutaneous adipose tissue is the primary cause of persistent adipose discomfort in the majority of cases. The national organization for rare disorders classifies it as a rare disease. Francis Xavier Dercum (1856-1931), an American neurologist in Philadelphia, was the first to describe this illness [1].
In 1888 [2] and 1892 [3], he wrote two articles about the illness and coined the medical term “adiposis dolorosa.” The same ailment was later observed at the same location by British physician Sir William Hale White (medical biography) and American physician James Meschter Anders (1854-1936) [4,5].
Although the clinical presentation of the syndrome may not always contain these related symptoms, the accompanying symptoms include fatigue or weakness, obesity or overweight, and various mental manifestations (e.g., sleep difficulties, emotional instability, sadness, and anxiety) [6]. DD was identified by the WHO as lipomatosis, which is not otherwise categorized (IV-E88.2) in ICD-10 DD [7].
Hansson et al., in 2012, established that DD is more commonly seen in obese women aged 35-50, but its aetiology remains unknown [8]. The most typical clinical manifestations of this condition include fatigue, depression, Weakness, and dementia. The pain complained by most patients usually involves adipose tissue within the extremities, abdomen, and buttocks, which is intermittent and fluctuates throughout the disease. Clinically, DD should be differentiated from other conditions such as lipedema, familial multiple lipomatosis, fibromyalgia, and adipose tissue tumours [9].
The same researchers have established a trend of obesity, painful flares, and hypertension in DD despite the scanty investigations regarding its presentation, pathophysiology, and drug therapy. The present study summarises recent data on the syndrome, highlighting its clinical presentation, differential Diagnosis, and drug management [9].
The main aim of this review is to answer the following questions:
• How often is DD associated with autonomic disorder?
• What is the pathogenesis of pain in DD?
Materials and Methods
Search strategy
From January 2000 to January 2025, we investigate the databases Embassy, Medline, Scopus, and PubMed Central using the following searches: “OR “OR “OR “treatment of Issac syndrome” OR “, OR “OR “. We searched the medical literature following the PRISMA guidelines. After removing duplicates, two reviewers (LDFIV and HFS) from each side screened titles and abstracts and evaluated the full texts of eligible articles based on the proposed inclusion criteria. Any disagreement between the reviewers involved in the literature search was resolved through discussion with all authors to reach a consensus.
Selection criteria
The following manuscripts were included in the systematic review:
Articles on Isaac syndrome, neuropathic pain, and Schwann cell disorder in ISA/NP, with detailed pathogenesis and/or drug therapy.
Exclusion criteria were as follows:
• Inaccessibility to full text.
• Articles with unclear pathogenesis.
• Lack of relevant clinicopathological data.
• Non-original studies (i.e., editorials, letters, conference proceeding, book chapters).
• Animal model studies.
• Non-/Spanish/Portuguese/English studies. The papers were thoroughly assessed, and duplicates were looked for.
Data extraction and quality assessment
All selected data were tabulated in an Excel electronic database. That information included pathogenesis, drug management, initial clinical presentation, evaluation of NP/IS after treatment, follow-up, and status at the latest evaluation. The quality of the studies included was categorized as good, poor, fair or reasonable, in agreement with the criteria of the National Institutes of Health.
Two reviewers (LDFIV and HFS) independently evaluated the articles, and the discussion resolved disagreements.
Statistical analysis
The primary objective of this study was to evaluate whether the pathogenesis/drug management of DD differs significantly among different ways of therapies. Without a comprehensive reference for the total number of DD cases, the prevalence of DD responding to drug therapy with associated autonomic manifestations was searched through a comprehensive review.
Statistical analyses were performed using XLSTAT (add on for Microsoft Excel, version 2021.4.1, Addinsoft SARL) and RStudio (version 4.3.1, https://www.rstudio. com/). Variations in continuous variables were assessed using the Mann Whitney U-test. The Chi-square test and Fisher’s exact were used to evaluate the association among categorical variables, as appropriate. We presented descriptive statistics for continuous variables as median (95% Confidence Interval (95% CI)). All situations were evaluated using the Kaplan-Meier method to identify relevant prognosticators. A model of multivariable Cox proportional hazards with a priori selection of covariates was used to check for independent prognostic effects.
To assess the therapy’s protective effect, we built a multivariable model that included only OMAS cases.
Results and Discussion
A total of 169 titles were selected from the literature. After removing duplicates and excluding records (N=53). 116 relevant articles were examined. 23 studies were unavailable for retrieving. After including six additional articles identified from citation searching (99), 43 was excluded for several reasons. A total of 56 records were identified from these searches. The resulting study titles were exported to Excel, and publications without including/ excluding criteria duplicates were removed, leaving 22 unique titles. We screened all remaining unique full-text articles, abstracts, and titles for eligibility. Titles were typically excluded if they were unavailable in Spanish, Portuguese, or English or irrelevant to 14. Articles relevant to NP/DD included the clinical presentation, management, treatment, pathogenesis, and treatment. A total of 10 articles remained following this initial screening. The authors then screened these articles by abstract. After further review, abstracts were excluded if they were irrelevant to the topic, outdated, or unavailable in Spanish, Portuguese, or English. XX abstracts were excluded, and four articles were for full-text review. Four were included for final review after screening the full-text articles for relevance. Finally, when we searched for association/DD/NP/Pathogenesis (DT), and no article was identified.
Series description and differences among groups
All the selected studies were relevant to the subject of this systematic review. None were randomized controlled trials or prospective studies; all the articles included were case reports and case series.
The total number of patients presenting NP/DD/documented pathogenesis/good response to DT was cero.
Median age was 17.5 (range 11-79) with significant differences between age groups (p<0.001). We did not find remarkable variations in gender (p=0.064), although females presenting OMAS were noticeably more frequent and slightly more prevalent.
A 41-year-old, separated, single-parent, right-handed male with no relevant medical background presented to us with a one-year history of muscle spasms and twitches (which he described as shock-like waves moving up his limbs multiple times a day, and were significantly worse if he was agitated), associated with urinary and bowel dysfunction (urinary retention and constipation), transient peripheral paraesthesia (viz., cramps, pins and needles of the upper and lower limbs) and slowly worsening vision. He also had nodules all over his body one of the lumps was biopsied previously and confirmed a lipoma. The Patient does not remember any preceding illness, surgical procedures, vaccines or trauma. No headaches, seizures or random falls were reported either. He admitted that he is under stress from his work.
Additionally, he is a single parent to one child (age 12 years). He follows a mixed diet and has sober habits. He also admitted to being very anxious recently.
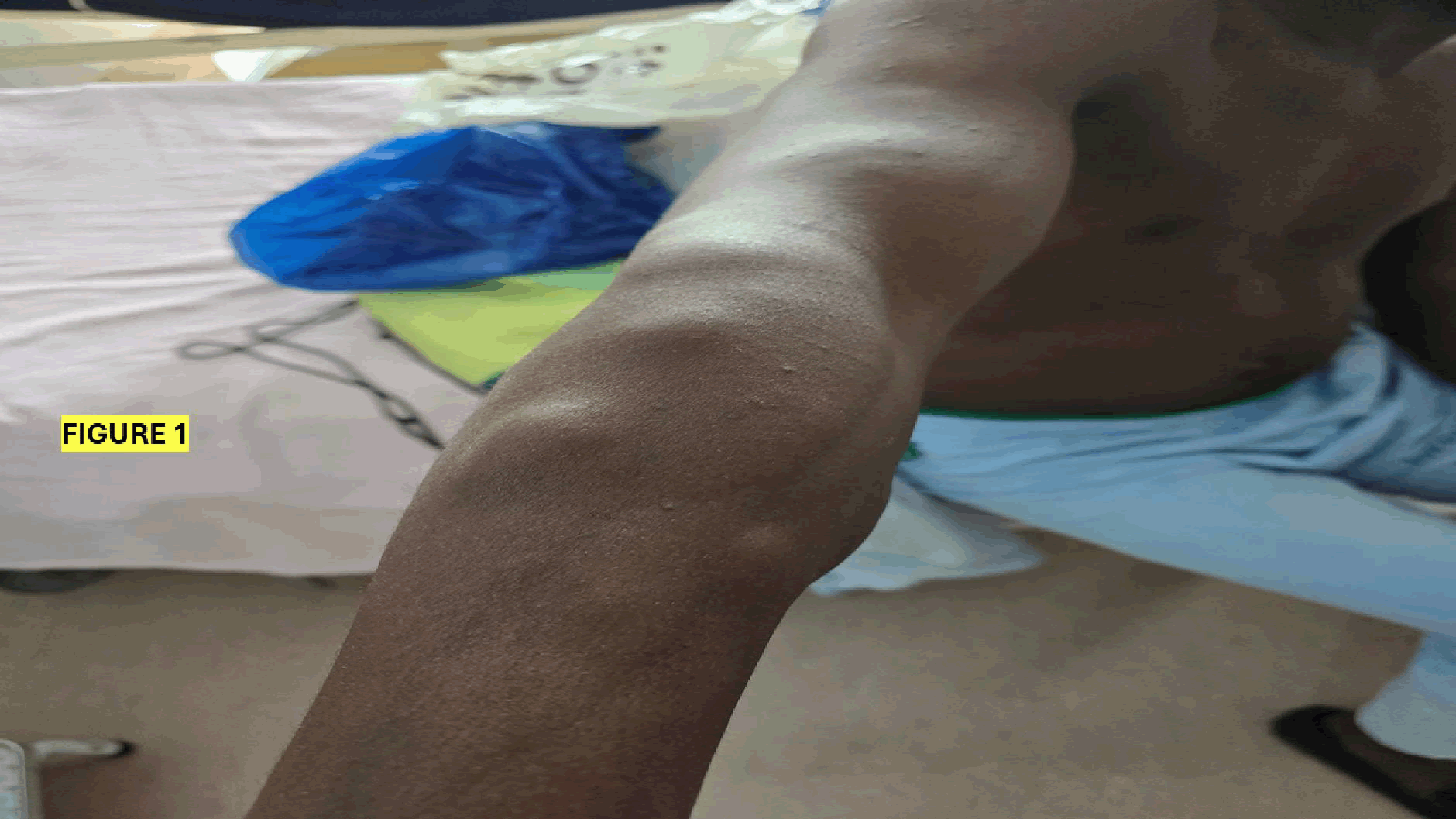
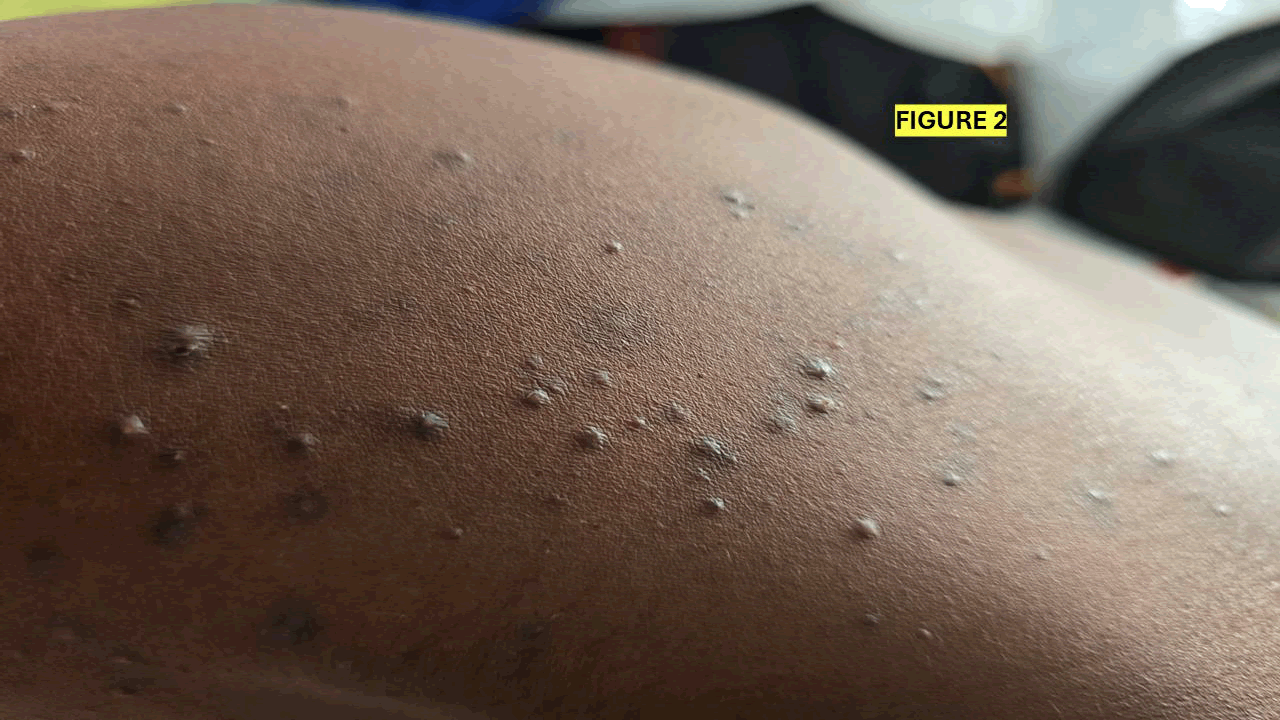
On examination, the patient was in fair general condition, with a BP of 93/64 mmHg, a heart rate of 79 beats/min, a temperature of 36.5°C, and SATS of 99% on room air. On local examination, two types of dermatological lesions were observed. Some nodular lesions of different sizes and painful to touch mainly at the upper limbs and small lesions at the shoulders bilaterally (Figures 1 and 2).
Figure 1: Prominent swelling along the forearm, suggestive of possible soft tissue or musculoskeletal abnormality. Further clinical evaluation is recommended
Figure 2: Multiple hyperpigmented and flesh-colored papules on the skin, characteristic of lichen planus or follicular-based dermatoses. Clinical evaluation is recommended for definitive diagnosis.
CNS: Awake, alert, oriented to time, place, person and situation. MSE 30/30, Cranial nerves: No focal neurological deficits. Motor: See tabulated below (Table 1).
| RUL | LUL | RLL | LLL | |
| Inspection | ||||
| Power: Proximal Distal |
5/5 5/5 |
5/5 5/5 |
5/5 5/5 |
5/5 5/5 |
| Tone | Normal | Normal | Spastic | Spastic |
| Reflexes | 2+ (normal) | 2+ (normal) | 3+ (brisk reflexes) | 2+ (normal) |
| Bilateral Babinski present | ||||
Table 1: Neurological examination findings of upper and lower limbs
Sensory system: Vibration, Joint Position Sense (JPS), light touch (posterior columns)-globally intact. Pinprick, pain/temp, crude touch (spinothalamic columns) globally intact. Coordination and Gait-nil dysmetria, nil Dysdiadochokinesia (DDK), nil ataxia. Normal gait.
SKIN and MSK: Multiple generalized lipomas all over the body, especially in the upper limbs (Figure 2), trunk and legs. Patients also had acne-like lesions on the face and the trunk (Figure 3),
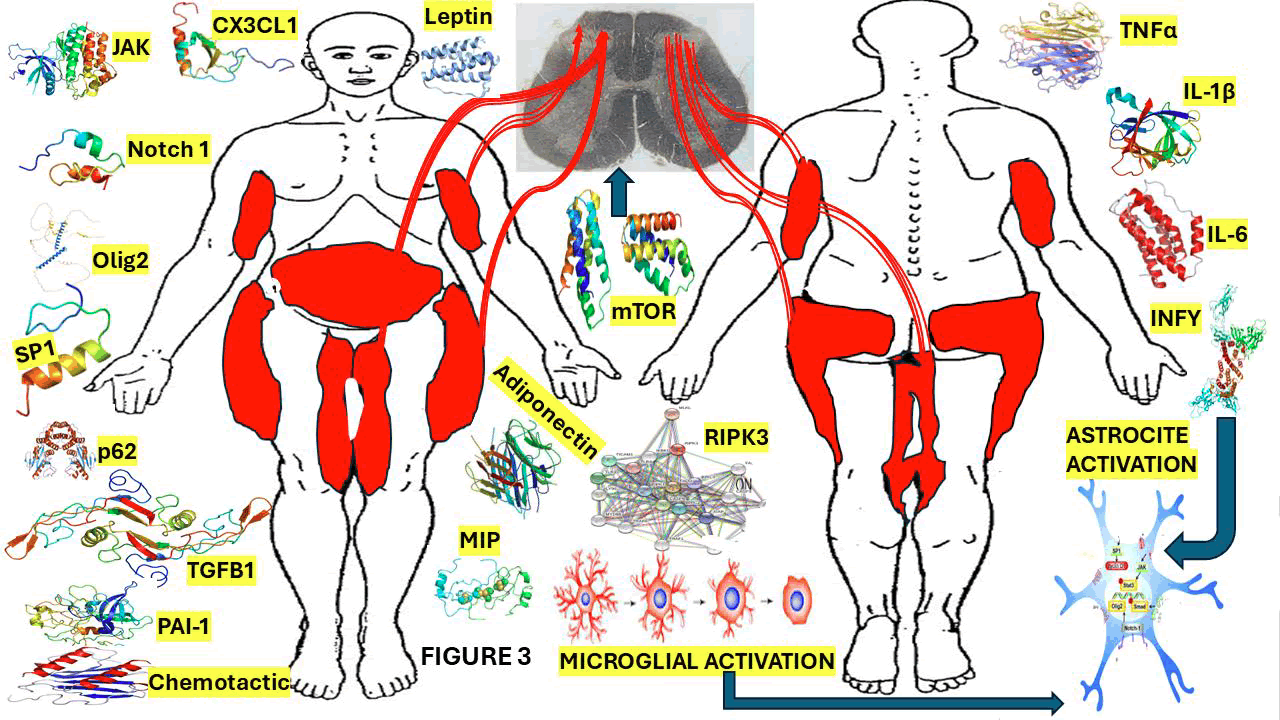
Figure 3: Graphical representation of the elements involved in the central mechanism of NP and showing the typical distribution of painful lesions in Dercum’s disease.
Note: TNF alpha, IL-6, IL-1b, Sp1 is a protein which acts as a transcriptional activator, regulating gene expression by binding to DNA and modulating whether a gene is turned off or on. It plays an important role in several biological processes including apoptosis, cell growth, and differentiation. Cyclin D1 is also a protein that plays a vital role in modulating cell cycle progression. Fibroblast Growth Factor (FGF) is a protein that stimulate the proliferation mainly of endothelial cells and that promote angiogenesis. Basic Fibroblast Growth Factor (bFGF), is a signaling protein encoded by the FGF2 gene, involved in various biological processes including tumor growth, angiogenesis, and wound healing. Epidermal Growth Factor (EGF) is a protein that stimulates cell growth and differentiation by binding to its receptor. Notch1 is a transmembrane receptor protein encoded by the NOTCH1 gene. Transforming Growth Factor-beta (TGF-β) is a multifunctional cytokine that plays a crucial role in cell growth, apoptosis, differentiation, and is involved in embryonic development, immune responses and wound healing. Janus Kinase (JAK) inhibitors used to treat chronic inflammatory disorders and increase the risk of major cardiovascular diseases like heart attack or stroke, blood clots in the lungs, cancer, serious infections and death when compared with TNF alpha inhibitors. STAT3, Signal Transducer and Activator of Transcription 3, is a protein that regulates gene expression by binding to DNA. It plays a crucial role in immune responses, cell growth, and differentiation, and it is implicated in the development of cancer. SMAD is an intracellular protein that plays a crucial role in regulating growth, cell development, and other biological processes. OLIG2, or Oligodendrocyte Transcription Factor 2, is crucial for the development and function of the CNS, particularly in the specification and differentiation of astrocytes and oligodendrocytes. ATP, or Adenosine Triphosphate, is the main energy source of cells. TSC2, also named tuberin, is a tumor suppressor gene that encodes a protein crucial for controlling cell growth and division associated to associated with tuberous sclerosis complex. mTOR, or mechanistic target of rapamycin, is a protein kinase that plays a vital role in regulating metabolism, cell growth, and survival. 18, HCH, RIP3, or receptor-interacting protein kinase 3, is a key protein involved in programmed cell death necroptosis, a pathway characterized by cell membrane rupture and inflammation. p62, also known as sequestosome 1, is a multifunctional protein involved in cellular homeostasis, autophagy, and signaling. Fractalkine (CX3CL1) is a transmembrane protein (type I) composed by 373 amino acid polypeptide chain. It’s also identified as a large cytokine protein member of the CX3C chemokine family. Plasminogen activator inhibitor 1: PAI-1 is a serine protease inhibitor (serpin) that works as the principal inhibitor of tissue-type Plasminogen Activator (tPA) and urokinase. Leptin is a hormone primarily produced by fat cells (adipocytes) that plays a crucial role in regulating appetite, and long-term energy balance. Adiponectin is a hormone mainly produced by adipose tissue, which plays a vital role in regulating the levels of glucose, insulin sensitivity, fatty acid breakdown, and inflammation. Macrophage Inflammatory Proteins (MIPs), specifically MIP-1β (CCL4), and MIP-1α (CCL3) are chemokines that are immune in the mechanism of immune responses by attracting specific immune elements to sites of inflammation and modulating inflammatory responses. Chemotactic proteins, also known as chemokines or chemotactic cytokines, are small, secreted proteins that stimulate the migration of leukocytes, towards a specific location by binding to cell surface receptors. They are classified into four subgroups based on the arrangement of cysteine residues in their structure: CC, CXC, CX3C, and XC.
Bloods: Na 137 mmol/L, K 4.1 mmol/L, urea 4.0 mmol/L, creatinine 99 umol/L, eGFR (MDRD formula) >60 mL/ min/1,73 m2. HbA1c 5.5%, Ca 2.38 mmol/L, PO4 1.05 mmol/L, Mg 0.85 mmol/L. Total protein 82 g/L, albumin 44 g/L, total bilirubin 10 umol/L, ALT 20 U/L, AST 32 U/L, ALP 73 U/L, GGT 36 U/L. CK 200 U/L. Total cholesterol 3.36 mmol/L, triglyceride 1.19 mmol/L, HDL cholesterol 0.87 mmol/L, calculated LDLC (Sampson) 1.98 mmol/L. CRP<1, RF<13, vit B12 328 pmol/L, serum folate 27.9 nmol/L. PSA 1.37, CEA<1.7, CA 125-6 kU/L, CA 19-9-6 kU/L, CA 15-3-15 kU/L. TSH 1.59 mIU/L, FT4 9.0 pmol/L, WCC 4.08, Hb 16.6 g/dL, platelets 268 x 109/L, ESR-10 mm/hr. INR-1, 06, D-Dimer-0.29. ANA (IFA)-negative, Aquaporin-4 IgG antibodies-negative, autoimmune encephalopathy Ab profile: NMDA, AMPA 1, AMPA 2, GABA-B receptor, CASPR2, LGI1 ALL negative. HIV-1/2 Ab/Ag ELISA Negative. Herpes simplex virus 1 and 2 IgG and IgM positive. CMV IgG-positive, IgM-negative, Toxoplasma gondii ELISA: IgG and IgM negative. Rubella IgG-positive, IgM-negative. T. pallidum antibodies-non-reactive. Cysticercosis ELISA-negative.
CSF: Glucose 3.3 mmol/L, protein 0.44 g/L, adenosine deaminase 0.0 U/L.
Appearance: Clarity is clear, colour before centrifugation is colourless, the colour of supernatant is colourless, and clots are absent.
Cell count: Polymorphs, lymphocytes, erythrocytes 0/uL.
India ink stain: Encapsulated yeasts not observed.
Gram stain: Organisms-no bacteria observed.
Cryptococcal latex antigen test: Negative.
TB-NAAT: GeneXpert MTB/Rif Ultra PCR.
Result: Mycobacterium tuberculosis complex not detected.
Bacterial cultures: No growth after 2 days.
FTA-ABS (Treponemal antibodies): Negative, Toxoplasma gondii ELISA IgG, IgM: Negative. Viral panel This meningitis screen is a multiplex PCR used to detect viruses that can cause meningitis.
Panel 1 (the Herpes virus panel) includes the following viruses: Cytomegalovirus, Epstein-Barr virus, and Varicella Zoster virus. Panel 2 (the acute meningitis panel) includes Adenovirus, Enterovirus, Mumps virus and echovirus. Oligoclonal bands: Type 1: No oligoclonal IgG in CSF or serum. Normal CSF. CTB: NAD. Based on the results above, the Patient was initiated on Acyclovir for the HSV. He also started on Orphenadrine (anticholinergic) for muscle spasms, carbamazepine and amitriptyline for peripheral neuropathy and lactulose for constipation. We also put him on clonazepam, thiamine, pyridoxine, folate, vitamin D and calcium carbonate. Dermatology was consulted, and he was given Doxycycline and Benzac Ac gel for the acne. Physiotherapy and occupational therapy reviewed him and started him on aggressive physical therapy. He was also seen by the psychology team and was initiated into psychotherapy, which he will undergo as an outpatient.
Comments and final remarks
We present a 41-year-old male no obese patient presenting with chronic neuromuscular symptoms of shock-like muscle spasms, lower limb spasticity, autonomic dysfunction, and systemic symptoms of multiple lipomas and acne like eruptions. Although initial investigations excluded infection, autoimmune encephalopathies, structural spinal disease, painful subcutaneous lipomas, exacerbation of symptoms with stress, and nonspecific neuropsychiatric symptoms (anxiety, fatigue) suggest adiposis dolorosa-a rare condition of painful adipose tissue.
The key diagnostic characteristics of AD are greater than 3 months of chronic pain in fatty masses and overweight/ obesity. Although not clinically obese, this patient’s lipomas, as confirmed by biopsy, chronic pain history, and subjective Weakness, are compatible with the disorder’s “minimal definition.” AD is 5-30 times more often in females than males, and its presentation is unusual but not unique. Associated symptoms such as constipation, tachycardia, and psychiatric disturbances also reflect this patient’s presentation. Still, the evidence of lower motor neuron signs (spasticity, Babinski) is not accounted for by classical adiposis dolorosa and raises the possibility of comorbid or additional pathology.
The previous summary case and discussion assess the potential for adiposis dolorosa as a cohesive diagnosis, showing concordant features (lipomas, chronic pain, systemic symptoms) and discordant features (gender, neurologic deficits) in multidisciplinary management.
We present a patient known to have DD who came to us without signs of obesity/overweight, which is not often previously described in the literature.
Fibromyalgia symptoms, like widespread pain and cognitive disorders, were not confirmed in our patient. Lipoedema was ruled out as he was already known to have lipomas, which are not seen in lipoedema, which mainly present as a disorder characterized by lack of pain and a diffuse increase in fat buildup.
Brief comments on the epidemiology of DD
A rare condition known as Dercum’s illness primarily affects people between the ages of 35 and 50. DD initially mostly affects postmenopausal women and is five to thirty times more common in women than in men. But according to a recent study, 85.7% of the chosen cases had DD prior to menopause [8]. The exact prevalence of DD at this time has not yet been established [8]. Most of the reported patients were Caucasian patients. A very scanty publication described the familial occurrence of DD. On the other hand, Cantu and collaborators suggested the autosomal dominant inheritance of the DD [10]. In contrast, Lynch and Harlan reported its familial occurrence in DD patients [11].
Notwithstanding the before-cited publication, all available medical articles indicate sporadic DD occurrence. DD may be heterogeneous, and inherited cases are a small subgroup of the syndrome [6].
Brief comments on clinical features and classification of DD
Pain, easy bruising, anxiety, fast heartbeat, shortness of breath, fatty deposits that are not affected by weight loss, depression, sleep disturbances, weakness, trouble focusing, diabetes, bloating, constipation, joint aches, and muscle aches are some of the symptoms of DD [6].
Hansson et al., mention rare symptoms described with DD [8], but none of which refer to neurological manifestations present in our case, which were associated with the painful f lares. Our patient reported that her flares had always been accompanied by urinary and bowel dysfunction (urinary retention and constipation), transient peripheral paraesthe sia (viz., cramps, pins and needles of the upper and lower limbs), and slowly worsening vision and those associated neurological manifestations were never reported before.
Our patient was diagnosed with type II form, as confirmed by his pain in the widespread distribution of his bilateral upper and lower extremities, even in the absence of a detectable lipoma.
Nevertheless, chronic pain in the adipose tissue further supported the Diagnosis of DD.
MRI of the lesions in DD is the gold standard for diagnostic imaging. Despite the characteristic features of type I, confirmatory signs can be seen on imaging only in types II, III, and IV.
As we depict in Figure 3, Hansson et al., [8] provided multiple descriptions and demonstrated that the most prevalent body areas with lipomas are the trunk, lower limbs, and upper limbs. However, in roughly one-third of DD patients, lipomas are seen on the scalp and neck, and in approximately one-fifth of instances, the face may be impacted. The thighs and buttocks are common sites for lipoma formation (about 70%).
After several painful adipose masses, being overweight or obese is regarded as the second cardinal sign. The majority of descriptions occur together with this ailment. The history states that Roux and Vitaut [12] identified two additional cardinal symptoms in 1901: Exhaustion or weakness and a number of psychological disorders.
Some investigators suggest that obesity and chronic pain are the cause of psychiatric manifestations, despite psychiatric manifestations associated with DD are not always found in all cases [7]. However, when they are present, it may include emotional instability and depression, sleep disturbances, less frequent cognitive impairment, and epilepsy.
Classification
The diagnostic criteria for DD include obesity or being overweight and generalized chronic pain in the adipose tissue for 3 months [6]. The classifications include four distinct varieties: type I-widespread painful adipose tissue with no apparent lipomas; type II-generalized, generalized nodular form; type III-localized, localized nodular form; and type IV-Juxta-articular form Table 2 [8-14].
There are fewer researchers who have suggested a different category of DD clinical presentation subtypes. Three illness forms are distinguished by the majority of categories; nonetheless, we suggested the previously mentioned in Table 2. The size of the lumps (larger in nearly all disease types and smaller in generalized, generalized diffuse form) and the distribution of fatty deposits (located near or within the joints, widespread versus localized) are taken into account in this classification. Descriptive findings serve as the basis for the classification, and reports of mixed and challenging-to-classify forms of DD were also made [15,16].
| Type | Description |
| Type I Generalized diffuse form | Widespread pain from fatty tissue, very small adipose deposits can be palpated in various parts of the body, but pain can occur in areas without detectable clear lipomas. |
| Type II Generalized nodular form | General pain in adipose tissue and intense pain in and around multiple lipomas which are localized in multiple parts of the body. |
| Type III Localized nodular form |
Pain in and around multiple lipomas. |
| Type IV Juxta-articular form |
Painful folds of fat located inside or very near big joints (the knee, hip or elbow). Solitary deposits of excess fat, for example at the medial aspect of the knee. |
Table 2: Classification of types of DD
Brief comments on diagnosis and differentiation
The diagnosis, which should be made by a comprehensive exclusion of possible diagnoses and a careful physical examination, is supported by strong clinical criteria. A doctor with extensive experience in painful illnesses or appropriate understanding of internal medicine, family medicine, or pain management should ideally make the clinical diagnosis. After the differential diagnoses have been ruled out, the final clinical diagnosis can be made.
Differential diagnosi
Panniculitis, endocrine disorders, fibromyalgia, lipoedema, main psychiatric disorders, multiple symmetric lipomatosis, familial multiple lipomatosis, and adipose tissue tumors should all be considered differential diagnosis.
After taking a history of present complaints and performing a proper physical examination describing painful lipomas and pain localized at the adipose tissue in an overweight person, DD should be differentiated from other painful conditions. Below, we will discuss the pathogenesis of the neuritic pain observed in DD and formulate some hypotheses. Table 3 summarises the most common conditions that should be differentiated from DD.
| Generalized diffuse and generalized nodular forms | Lipoedema Lymphedema Fibromyalgia Proteus syndrome Familial multiple lipomatosis Panniculitis Weber-Christian disease Cushing’s syndrome Frohlich’s syndrome Progressive lipodystrophy Hypothyroidism Multiple symmetric lipomatosis |
| Nodular forms | Benign symmetric lipomatosis (Madelung’s syndrome, Launois-Bensaude syndrome) Familial multiple lipomatosis Neurofibromatosis type 1 Adenolipomatosis Multiple endocrine neoplasia I Adipose tissue tumours Myoclonic Epilepsy with Red Ragged Fibres (MERRF) |
Table 3: Differential diagnosis of DD
Based on our findings from searching the medical literature, fibromyalgia, panniculitis, lipoedema, and lymphedema present more difficulties differentiating from DD.
Fibromyalgia is the hardest to distinguish from DDD because of its widespread muscular pain associated with psychiatric manifestations and the detection of painful lipomas. However, lipoedema is luckily restricted to the lower limbs, which are distinguished by symmetrical and uniform fat deposition on both sides [17].
Panniculitis is usually associated with local inflammation of the lower limbs; some of the nodules heal while others develop apart from being erythematous. Lymphedema responds to compression or lymphatic massage resultsbecause of the accumulation of protein-rich interstitial fluid within the skin, which is massively localized and is hard to differentiate from DD [18,19].
Other symptoms to be considered for a proper clinical diagnosis of DD include fatty deposits, depression, sleep disturbances, easy disability, impaired memory, anxiety, rapid heartbeat, diabetes, bloating, difficulty concentrating, dyspnoea, constipation, fatigue, weakness and joint aches [20].
Brief comments on the aetiology of DD
Rasmussen et al., hypothesized that the aetiology of DD may be a lymphovascular condition and proposed a possible combination of abnormal adipose tissue deposition and dysfunctional lymphatic structure [21].
According to Campen et al., [22] DD may be caused by an inflammatory process that releases neuropeptides linked to pain. There have also been suggestions of a traumatic aetiology [23].
Rasmussen et al., [21] claim that lymphovascular damage can result in a lack of lymphatic transport, which can cause lymphatic vessels to discharge dysfunctionally, which is the cause of DD. Some unconfirmed causes include mechanical pressure on the peripheral nerves, CNS dysfunction, adipose tissue disease, and trauma. However, none of them have been confirmed as an aetiology for DD.
In 1989, Dalziel [24] proposed that the Autonomous Nervous System (ANS) modulates pain in DD based on the efferent function of the sympathetic nervous system, and sympathectomy improved NP.
It has been supported by abnormal connections between sensory nerves and autonomic fibres in the periphery, leading to abnormal autonomic signalling to the spinal column, activating the pain fibres [24]. Moreover, cases with DD could have incremented sympathetic activity induced by nociceptive impulses. Moreover, visceral pain may be produced by the autonomic nervous system, and substances that cause visceral pain may also be able to cause discomfort in adipose tissue [24].
Endocrine dysfunction
In his second original article, Dercum [3] considered endocrine dysfunction as the aetiology of diseases based on his finding of the atrophied thyroid gland. Waldorf [25] proposed hypophyseal dysfunction as the leading cause of DD.
Furthermore, Winkelman and Eckel [26] found endocrines abnormalities in sixteen autopsies of patients affected with DD. Among their findings, the thyroid gland was the most affected, followed by the pituitary gland, sex glands, adrenal gland and pancreas, in that order.
However, the endocrine involvement in DD was completely ruled out in 1952 [27].
Our review of the modern medical literature shows no evidence of endocrine abnormalities in DD.
Mechanical pressure on the nerves
The theory of NP based on cases where growing fatty masses stretching or pressuring on peripheral nerves has never been confirmed histopathologically [28].
Adipose tissue dysfunction
Dysfunctional local lipid metabolism is another aetiology that Juhlin and his colleagues proposed for DD in 1971. Based on defective fatty acid biosynthesis found in two patients, these authors believed that a deficit in forming monounsaturated fatty acids affects these structures in cases of DD. These authors also found a disproportion of monounsaturated fatty acids, which was remarkably higher in the patients with DD compared with a control group, but that statement has never been proved further [29].
Furthermore, other investigators found that DD patients have lower resting energy expenditure according to their body mass (kg) than group controls. However, based on our systematic review, we concluded that there is insufficient evidence to establish that adipose tissue disorder is an aetiology of DD.
Inflammation and autoimmune aetiologies
Despite several investigators proposing inflammation as an aetiology for DD [20], all inflammatory markers, including ESR and CRP, are usually normal. However, these markers have been found positive [20]. Also, markers for autoimmune disorders, including antinuclear antibodies, anticardiolipin antibodies, rheumatoid factor, antibodies against native DNA, perinuclear anti-neutrophil cytoplasmic antibodies, and cytoplasmic anti-neutrophil cytoplasmic antibodies are reported negative in DD [20].
We hypothesized that although most proinflammatory molecules are not usually increased in DD, they are strongly represented in the NP pathogenesis accompanying DD. Therefore, we will document it below, including our graphical representation of plasminogen activator inhibitor-1, Monocyte Chemotactic Protein (MCP), leptin, adiponectin, Interleukin (IL)-1β, IL-6, Tumor Necrosis Factor (TNF)-α, and Macrophage Inflammatory Protein (MIP)-1α. However, there have been reports of noticeably reduced MIP-1β activity, and a tendency toward higher IL 13 and lower fractalkine levels was discovered [20].
We hypothesized the role of fractalkine in the pathophysiology of NP, as we will discuss below.
We hypothesized that fractalkine could promote resistance to opioid analgesia by occupying the free nerve-ending receptors. Therefore, the NP will be more sustainable, which has been supported by other authors under different conditions long ago [30].
Trauma-induced DD
Although traumatic lesions have been included in the list of aetiologies for DD, only four cases of DD associated with spontaneous pain have been reported [31,32].
Therefore, it represents a scanty involvement of peripheral nerve trauma in the pathogenesis of this condition and a clear-cut misunderstanding of the real pathogenesis of NP in DD. Most of the evidence described in the reported case is circumstantial. We hypothesized that local nerve compression can explain local pain in some cases, but this statement does not cover the real pathogenesis of the central component. Therefore, this mechanism will be discussed below, and a graphical representation will be released.
Brief comments on management and drug therapy
Following the use of lidocaine, methotrexate, infliximab, interferon α-2b, liposuction, analgesics, corticosteroids, calcium-channel modulators, and rapid cycling hypobaric pressure, appropriate treatment and medication therapy have resulted in some pain relief in DD patients. DD should be treated in multidisciplinary groups that specialize in chronic pain because none of the treatments have produced exceptional outcomes or long-lasting, full pain relief.
In many cases, administering local anaesthetic injections is a relatively non-invasive and safe therapy modality that properly treats pain in cases with DD [1]. In 2019, Wipf and collaborators reported good results administering bile acid medications to a group of cases presenting DD. Injecting 2 mg/cm2 of deoxycholic acid (cytolytic medication) into subcutaneous fat, where adipose tissue causes pain, might provide good improvement. However, side effects such as nodules, headache, swelling, oedema, pruritus, and paresthesia can be observed. However, this therapy requires further confirmation [33]. On top of that, other authors suggest methotrexate, interferon α-2b, liposuction, analgesics, infliximab, calcium-channel modulators, rapid cycling hypobaric pressure, intralesional lidocaine, and corticosteroids plus psychotherapy and consultation with chronic pain specialists [34,35].
To perform delayed Split-Thickness Skin Graft (STSG) after interval use of wound Vacuum-Assisted Closure (VAC) following dermolipectomy was recommended for the first time in 2020 by Opoku-Agyeman and collaborators with acceptable results in a 77-year-old female [36]. Other surgical management include liposuction [37-42].
However, liposuction is ineffective in long-standing disease due to fibrosis [43,44].
The therapy strategy in cases with DD is decided individually by the attending doctor because only a few publications have been released, and most of them report f indings from single cases without statistical value.
Hansson et al., [45] reported good results related to pain release in 53 patients whose liposuction was performed. The same group of investigators also found that these patients had a better quality of life after liposuction [20].
A few reported patients underwent surgical procedures. Lipectomy or dermo lipectomy ameliorated the disorder, even in the juxta-articular form [42,43].
Therapy for subcutaneous adipose tissue based on massage of fascia, muscle, and deep fat tissue has been used to reduce lipomas in DD [42].
Invasive therapy focuses on local anaesthesia, mainly in cases refractory to nonsteroidal anti-inflammatory drugs. Common applications for lidocaine include transdermal methods, intravenous infusions, and intralesional injections [47,48].
Although it hasn’t been verified, these authors reported that the mechanism of action involved inhibiting electrical conduction in peripheral nerves and the impact of sympathetic activity. Transcutaneous frequency rhythmic electrical stimulation was shown to have significant advantages by other writers, demonstrating that it was a safe and efficient therapeutic technique [49,50].
Currently, pain relief with narcotic analgesics andnonsteroidal anti-inflammatory drugs has been unpredictable. However, significant pain relief has been obtained by administrating a variety of therapeutic drugs, including methotrexate, Interferon α-2b, infliximab, and calcium channel modulators (pregabalin and oxcarbazepine). However, the mechanism and significance of pain relief from these drugs remain unclear, and only a few anecdotic publications regarding good response to interferon α-2b, pregabalin, corticosteroids, calcium-channel modular, metformin, methotrexate, and infliximab have been released [20,51-53]. Furthermore, a case report has shown that infliximab decreases NP in patients with neurosarcoidosis through tumour necrosis factor inhibition [52].
The administration of deoxycholic acid injections to manage lipomas in a patient with DD has been reported to have a good response [54], but this therapeutic procedure needs to be evaluated.
Because DD is a chronic recurrence disorder characterized by painful lipomas throughout the body and obesity, we consider that all drug therapy should be based on an analysis of the severity of symptoms and type of the disease. Unfortunately, there is no curative treatment for DD. However, other therapy modalities have been considered, including surgical approaches.
Surgical intervention
Some studies reported short-term effectiveness after performing liposuction and lipoma resection. Although liposuction is strictly contraindicated in recurrent lipomas, this procedure has shown some benefits when the sensory nerves are included [55].
Sub-anaesthetic therapy
As we previously cited, lidocaine has been administered as a topical agent and IV infusion to treat NP due to DD for a long time. Some authors reported 60% sustained pain reduction using lidocaine and 5% transdermal patches in some cases [56]. Lidocaine has the ability to boost sympathetic nervous system activity by acting on sodium channels in peripheral nerves [20,57].
Ketamine, on the other hand, has long been used to treat NP because of its ability to lessen pain intensity by blocking N-methyl-D-aspartate receptors.
Administration of ketamine is recommended as follows: 500 mg of ketamine in a 500 mL bag of 0.9% NaCl. A 60 mg slow IV push to be followed by 60 mg/h increased every 15 min by 10 mg/h for a maximum dose of 150 mg/h [57].
Subanaesthetic ketamine infusions used to treat refractory pain syndromes have shown acceptable responses despite reported side effects such as hypertension, sedation, hallucination and confusion [56]. In the past, the protocol for ketamine infusion studies has been very heterogeneous, leading to unconfident therapeutic guidelines. However, now we have a better available guideline approved by the American Society of Regional Anaesthesia and Pain Medicine, the American society of Anaesthesiologists and the American Academy of Pain Medicine, which offer better standardization guidelines for the administration of ketamine infusion [58,59].
Physical modalities
Several investigators have described manual lymphatic massage for patients presenting outflow lymphatic obstruction associated with malignant growth and lipomatous growths of DD leading to NP. The same researchers recommend applying this massage twice weekly [20].
Electrocutaneous stimulation
Because pain is one of the most important symptoms of DD, which is often invalidating and resistant to drug therapy, including potent analgesic, other investigators have proposed the administration of a Frequency Rhythmic Electrical Modulation System (FREMS) for pain relief based on their findings from nine patients [60]. The same authors documented the presence of the mitochondrially encoded tRNA-Lysine (MT-TK) m.8344A>G variant found in patients with multiple symmetric lipomatosis. It was excluded in all groups where they also observed different genes belonging to signalling pathways. The cell cycle and proliferation elements are phosphoinositide 3-kinase/AKT/ mTOR and MAPK/ERK. [55]. Finally, it is concluded that FREMS is a valuable procedure to alleviate NPs and improve the overall quality of life in cases presenting with DD.
In one case treated by Martinenghi and colleagues, DD improved following transcutaneous Freshen treatment, which was administered in four cycles of thirty minutes each for six months [61].
The advantage of MC5-A Calmare (another cutaneous electrostimulation modality) over FREMS has not been demonstrated. In addition, other inconveniences, such as difficult access to a Calmare machine, reproducibility of electrode placement to achieve “zero pain,” operator training, and insurance coverage, have been reported [55].
Perineural injection/prolotherapy
Perineural Injection Therapy (PIT) involves subcutaneous injection of dextrose solution into tissues surrounding an inflamed nerve to reduce neuropathic inflammation. Its primary goal is to alleviate the inflammatory process over the superficial branches of peptidergic peripheral nerves. Pain relief is fast, but to get sustainable effects, repeating the procedure several times is strong recommended [55].
Hypobaric pressure therapy
Some authors propose administering cycling hypobaric pressure to decrease the pain associated with peripheral oedema in cases with DD [62]. However, other investigators have not replicated their good results to date.
Acupuncture
Although we could not find a single publication regarding the use of Acupuncture (Ac) in cases of DD, it is well known that this Chinese procedure has been used to relieve chronic pain conditions. Some authors investigated patients presenting chronic pain due to peripheral joint arthritis and other conditions treated with Ap, obtaining an excellent response [63-65]. Because DD is a scarce condition, determining how a large cohort of patients presenting DD under acupuncture therapy will respond to Ap will be a challenging confirmation to reach.
Interferon α-2b
Because interleukin-1 and tumour necrosis factor are involved in cutaneous hyperalgesia, some authors proposed using interferon α-2b to treat patients with DD due to its antiviral effect and its involvement in the production of endorphins to interfere with the production of proinflammatory elements [66]. Unfortunately, this modality of treatment has not been implemented at the proper times to prove its effectiveness.
Corticosteroids
Another drug therapy used to treat cases of DD showing some improvement is systemic corticosteroids (prednisolone), which is based on its capacity to inhibit the effects of histamine, bradykinin, serotonin, and prostaglandins [20]. Other investigators found worsening pain in cases presenting juxta-articular DD [[67]], probably because DD is not an inflammatory disease.
Calcium-channel modulators
Some calcium-channel modulators have been prescribed to treat DD, such as pregabalin (AED) [[20]] and oxcarbazepine (AED) [[20]], based on their properties to inhibit the activation of neuronal calcium channels and excitatory amino acids (for central sensitization) and relief NP [[68]].
D-thyroxine
Because the first statement on the aetiology of DD is related to endocrine diseases, some authors administered D-thyroxine to this process without any response [[20]].
New hypotheses on neuropathic pain related to DD
Some authors describe fine pain as “an unpleasant emotional and sensory experience resembling or associated with potential or actual tissue damage” [[69]]. Identifying painful stimuli is essential for a species’ survival and well being, as it prevents the development of injured tissue and compromises its long-term functioning.
The perception of nociception involves all the physiological processes integrating real tissue damage or a painful stimulus to signal potential. Therefore, pain perception relies on the sensitivity of altered tissue, which is projected to CNS structures before being integrated into memory and emotional networks. There are three types of nociceptive stimuli: chemical, mechanical, and thermal.
Nociceptive signals are initiated by nociceptors and convey a pain sensation to the CNS [69]. These nociceptors are usually weakly myelinated or unmyelinated, known as Aδ or C fibres. In DD cases, we hypothesized that pain signal transduction is initiated at the peripheral nerve endings of nociceptors through a membrane depolarization depolarization called a receptor potential. In these patients, this depolarisation produces action potentials that propagate the information from the peripheral terminals to the posterior horn of the cord. From here, it is integrated and transmitted to the brain via the spinothalamic tracts to the primary sensory cortex in the parietal lobe.
The Transient Receptor Potential (TRP) channel family gathers throughout species receptors mainly permeable to Na+ and Ca2+ ions and then divided into six subfamilies: Canonical (TRPC), ankyrin (TRPA), melastatin (TRPM), polycystin (TRPP), vanilloid (TRPV), and mucolipin (TRPML) [69]. Some of them are activated in nociceptors and thus involved in the transduction of a nociceptive stimulus.
Their activation in the sensory afferents induces membrane depolarisation, which ultimately generates a painful stimulation in the CNS [69].
Neuropathic Pain (NP) is a condition that results from dysfunctional central and peripheral pain conduction pathways characterized by chronic spontaneous pain, hyperalgesia, and allodynia, often perceived as a refractory pain syndrome [70].
The central part pathway includes the posterior horn of the spinal cord, which is a gray matter that receives sensory information from the peripheral sensory receptor at the periphery and sends it to the sensory brain cortex via ascending pathways. Figure 3 which shows most of components of our hypothesis related to the pathophysiology of NP in DD involving the Mg/As expression in the posterior horn of the SC.
Dong et al., [70] also found that following chronic constriction injury surgery, the mammalian Target of Rapamycin (mTOR) increased in As and As’ expression within the spinal cord. This point is appropriated to highlight some aspects related to the mTOR, which is a member of the family protein kinases encoded by the MTOR gene with the capacity to regulate several cellular processes such as cell survival, protein synthesis, cell growth, cell proliferation, cell motility, autophagy/apoptosis (by participating in multiple signalling pathways in the body) transcription, expression of insulin receptors and insulin-like growth factor 1 receptor; mTORC2 has also been involved in the maintenance and control of the actin cytoskeleton; mTOR signalling pathway is also implicated with arthritis, insulin resistance, cancer, osteoporosis among many other disorders.
We hypothesized that NP in patients presenting DD, the pharmacological inhibition of mTOR reversed CCI-in duced neuroinflammation and hyperalgesia. Otherwise, the knockdown of astrocytic mTOR may recover the down regulation of spinal glutamate metabolism-related protein activity, underscoring the crucial role of mTOR in modu lating this pathway.
The same authors [70] reported that overexpression of mTOR, achieved through intrathecal administration of TSC2-shRNA, causes an upregulation of Receptor Interacting Protein 3 (RIP3), which can be applied to cases of DD.
On the other hand, pharmacological inhibition blockage of RIP3 might eliminate the mTOR-induced as activation while not modulating mTOR activation, which can control the RIP3 expression in As through ITCH-mediate by autophagy-dependent degradation. Despite a few effective treatments for NP, we hypothesized that the results of drug therapy of NP in cases with IS would improve if we find all the necessary knowledge to modulate the link between mTOR and RIP3, producing an expression, leading to a proper peripheral and central sensitization. The sustainability of chronic NP in cases with IS should be considered because of an enhanced neuronal reactivity in central pain pathways after painful damage/NI, which drives central sensitization.
On the other hand, activating Mg and As triggers the release of proinflammatory molecules, worsening NI. Other investigators support it by documenting the involvement of as in the development and maintenance of NP [71-73].
Fang et al., support the previous postulate by demonstrating that pain sensibility decreases when astrocyte activity within the spinal cord is inhibited [74].
Unfortunately, after completing our systematic review on this issue, we could not find the appropriate information to determine how mTOR is involved in as activation leading to NP. However, other authors have documented the confirmation that inhibition of mTOR alleviates demyelination and NI in globoid cell leukodystrophy.
Conclusion
To our knowledge, this is the first reported case presenting DD and a combination of muscle spasms and twitches (which he described as shock-like waves moving up his limbs multiple times a day, and were significantly worse if he was agitated), associated with urinary and bowel dysfunction (urinary retention and constipation), transient peripheral paraesthesia (viz., cramps, pins and needles of the upper and lower limbs) and slowly worsening vision. Based on the systematic review of the medical literature we also released some novel hypotheses on the pathogenesis of NP in patients presenting DD. Obviously further investigations will support or reject our proposals.
Author Contributions
Both investigators have read and agreed to the published version of the manuscript.
Conflict of Interest Statement
The authors declare they have no conflicts of interest.
Funding Information
The authors received no funds to perform the present re search.
Ethics Statement
The study was conducted using the principles of the Helsinki Declaration, the Italian and US privacy and sensitive data laws, and the internal regulations for retrospective studies of the Otolaryngology Section at Padova University and Brescia University.
Informed Consent Statement
We obtained the informed consent from the case involved in the study.
Data Availability Statement
The corresponding author will make the raw data supporting this article’s conclusions available upon request.
Acknowledgments
The authors thank Prof Thozama Dubula, Head of Department on Internal Medicine and Therapeutic for his unconditional support in the management of this patient.
Therefore, this study recommends starting with the family environment to overcome the circulation of alcoholic beverages. Parental and family supervision of children and siblings, as well as the surrounding environment, can help ensure the success of this local regulation. Collaboration with the police is necessary as government partners in overcoming the circulation of alcoholic beverages, and prevention through routine patrols must be prioritized.
References
- D.A. Patel, K.G. Swan, Francis Xavier, Dercum: A man for all seasons, Ann Clin Transl Neurol, 1(2014):233-237.
[Crossref] [Google Scholar] [PubMed]
- F.X. Dercum, A subcutaneous connective tissue dystrophy of the arms and back, associated with symptoms resembling myxedema, Univ Med Mag (Philadelphia), 1(1888):1-11.
[Crossref]
- F.X. Dercum, Three cases of a hitherto unclassified affection resembling in its grosser aspects obesity, but associated with special nervous symptoms adiposis dolorosa, Am J Med Sci, 101(1892):521-523.
- J.M. Anders, A Textbook of the Practice of Medicine, W.B. Saunders & Company, Philadelphia, US state. (1897).
- W.H. White, A case of adiposis dolorosa, Br Med J, 2(1899):1533-1534.
[Crossref] [Google Scholar] [PubMed]
- O.A. Housni, C. Boufeas, V. Slane, Dercum’s disease: The clinical presentation, diagnosis, radiological findings, and treatment of a rare, debilitating inflammatory disorder, HCA Healthc J Med, 5(2024):171-174.
[Crossref] [Google Scholar] [PubMed]
- E.J. Kucharz, M.K. MÄdrek, J. Kramza, M. Chrzanowska, P. Kotyla, Dercum’s disease (adiposis dolorosa): A review of clinical presentation and management, Reumatologia, 57(2019):281-287.
[Crossref] [Google Scholar] [PubMed]
- E. Hansson, H. Svensson, H. Brorson, Review of Dercum’s disease and proposal of diagnostic criteria, diagnostic methods, classification and management, Orphanet J Rare Dis, 7(2012):23.
[Crossref] [Google Scholar] [PubMed]
- J.C. Cook, G.P. Gross, StatPearls NCBI Bookshelf version. Stat-Pearls Publishing. Florida, US State. 2023.
- J.M. Cantu, E. Ruiz-Barquin, M. Jimenez, Autosomal dominant inheritance in adiposis dolorosa (Dercum’s disease), Humangenetik, 18(1973):89-91.
[Crossref] [Google Scholar] [PubMed]
- H.T. Lynch, W.L. Harlan, Hereditary factors in Adiposis Dolorosa (Dercum’s Disease), Am J Hum Genet, 15(1963):184-190.
[Google Scholar] [PubMed]
- J. Roux, M. Vitaut, Maladie de Dercum (Adiposis dolorosa), Revue Neurol (Paris), 9(1901):881-888.
- A. Lam, W. Aukerman, B. Winegarden, S. Morrissey, Lurking under the surface: Dercum's disease, Cureus, 13(2021):e17649.
[Crossref] [Google Scholar] [PubMed]
- E. J. Kucharz, M. Kopec-MÄdrek, J. Kramza, M. Chrzanowska, P. Kotyla, Dercum’s disease (adiposis dolorosa): A review of clinical presentation and management, Reumatologia, 57(2019):281-287.
[Crossref] [Google Scholar] [PubMed]
- H.T. Lynch, W.L. Harlan, Hereditary factors in adiposis dolorosa (Dercum's disease), Am J Hum Genet, 15(1963):184-190.
[Google Scholar] [PubMed]
- S.G. Chen, S.D. Hsu, T.M. Chen, H.J. Wang, Painful fat syndrome in a male patient, Br J Plast Surg, 57(2004):282-286.
[Crossref] [Google Scholar] [PubMed]
- D.W. Buck, K.L. Herbst, Lipedema: A relatively common disease with extremely common misconceptions, Plast Reconstr Surg Glob Open, 4(2016):e1043.
[Crossref] [Google Scholar] [PubMed]
- J.M. Petscavage-Thomas, E.A. Walker, S.A. Bernard, J. Bennett, Imaging finding of adiposis dolorosa vs. massive, localized lymphedema, Skeletal Radiol, 44(2015):839-847.
[Crossref] [Google Scholar] [PubMed]
- E. Hansson, H. Svensson, H. Brorson, Review of Dercum’s disease and proposal of diagnostic criteria, diagnostic methods, classification and management, Orphanet J Rare Dis, 7(2012):23.
[Crossref] [Google Scholar] [PubMed]
- J.C. Rasmussen, K. L. Herbst, M. B. Aldrich, C. D. Darne, I. C. Tan, et al. An abnormal lymphatic phenotype is associated with subcutaneous adipose tissue deposits in Dercum’s disease, Obesity (Silver Spring), 22(2014):2186-2192.
[Crossref] [Google Scholar] [PubMed]
- R. Campen, H. Mankin, D.N. Louis, Familial occurrence of adiposis dolorosa, J Am Acad Dermatol, 44 (2001):132-136.
[Crossref] [Google Scholar] [PubMed]
- D. Hao, A. Olugbodi, N. Udechukwu, A.A. Donato, Trauma-induced adiposis dolorosa (Dercum’s disease), BMJ Case Rep, 2018; 2018.
[Crossref] [Google Scholar] [PubMed]
- K. Dalziel, The nervous system and adipose tissue, Clin Dermatol, 7(1989):62-77.
[Crossref] [Google Scholar] [PubMed]
- N.W. Waldorp, An original clinical interpretation of Dercum’s disease (adiposis dolorosa), Endocrinology, 8(1924):51-60.
- N. Winkelman, J.L. Eckel, Adiposis dolorosa (Dercum’s disease): A clinicopathologcal study, JAMA, 85(1925):1935-1939.
- W.A. Steiger, H. Litvin, E.M. Lasche, T.M. Durant, Adiposis dolorosa (Dercum’s disease), N Engl J Med, 247(1952):393-396.
- B.A. Mella, Adiposis dolorosa, Univ Mich Med Cent J, 33(1967):79-81.
- R. Blomstrand, L. Juhlin, H. Nordenstam, R. Ohlsson, B. Werner, et al. Adiposis dolorosa associated with defects of lipid metabolism, Acta Derm Venereol, 51(1971):243-250.
[Crossref] [Google Scholar] [PubMed]
- K.L. Herbst, A.D. Coviello, A. Chang, D.L. Boyle, Lipomatosis-associated inflammation and excess collagen may contribute to lower relative resting energy expenditure in women with adiposis dolorosa, Int J Obes (Lond), 33(2009):1031-1038.
[Crossref] [Google Scholar] [PubMed]
- J.H. Hall, C.E. Walbrach, Adiposis dolorosa with report of three cases, Am J Med Sci, 128(1904):218-322.
- G. Margherita, Considerations on a case of post-traumatic adiposis dolorosa associated with a pathologic fracture, Rass Neuropsichiatr, 18(1964):211-218.
- A. Wipf, S. Lofgreen, D.D. Miller, R.S. Farah, Novel use of deoxycholic acid for adiposis dolorosa (Dercum disease), Dermatol Surg, 45(2019):1718-1720.
[Crossref] [Google Scholar] [PubMed]
- E.J. Kucharz, M. Kopec-MÄdrek, J. Kramza, M. Chrzanowska, P. Kotyla, Dercum’s disease (adiposis dolorosa): A review of clinical presentation and management, Reumatologia, 57(2019):281-287.
[Crossref] [Google Scholar] [PubMed]
- M. A. Baig, An unusual presentation of Dercum’s disease to the emergency department, Oxf Med Case Reports, 2023(2023):075.
[Crossref] [Google Scholar] [PubMed]
- J. L. Opoku-Agyeman, L. Coffua, J. Simone, T. Hanley, A. Behnam, Exophytic adiposis dolorosa (Dercum’s Disease) of the thigh: A case report, Cureus, 12(2020):e7282.
[Crossref] [Google Scholar] [PubMed]
- E. Hansson, H. Svensson, H. Brorson, Liposuction may reduce pain in Dercum’s disease (adiposis dolorosa), Pain Med, 12(2011):942-952.
[Crossref] [Google Scholar] [PubMed]
- M. De silva, M.J. Earley, Liposuction in the treatment of juxta-articular adiposis dolorosa, Ann Rheum Dis, 49(1990):403-404.
[Crossref] [Google Scholar] [PubMed]
- A.J. DeFranzo, J.H. Hall, S.M. Herring, Adiposis dolorosa (Dercum’s disease): liposuction as an effective form of treatment, Plast Reconstr Surg, 85(1990):289-292.
[Google Scholar] [PubMed]
- H. Lemont, J. Picciotti, J. Pruzansky, Dercum’s disease, J Am Podiatry Assoc, 69(1979):389-391.
[Crossref] [Google Scholar] [PubMed]
- T.J. Bonatus, A.H. Alexander, Dercum’s disease (adiposis dolorosa). A case report and review of the literature, Clin Ortho Relat Res, 205(1986):251-253.
[Google Scholar] [PubMed]
- J.L. Held, J.A. Andrew, S.R. Kohn, Surgical amelioration of Dercum’s disease: A report and review, J Dermatol Surg Oncol, 15(1989):1294-1296.
[Crossref] [Google Scholar] [PubMed]
- U. Wollina, A. Goldman, B. Heinig, Microannular tumescent liposuction in advanced lipedema and Dercum’s disease, G Ital Dermatol Venereol, 145(2010):151-159.
[Google Scholar] [PubMed]
- W. Schmeller, I. Meier-Vollrath, Tumescent liposuction: A new and successful therapy for liphedema, J Cutan Med Surg, 10(2006):7-10.
[Crossref] [Google Scholar] [PubMed]
- E. Hansson, H. Svensson, H. Brorson, Liposuction may reduce pain in Dercum’s disease (adiposis dolorosa), Pain Med, 12(2011):942-952.
[Crossref] [Google Scholar] [PubMed]
- M. Ibarra, A. Eekema, C. Ussery, Subcutaneous adipose tissue therapy reduces fat by dual X-ray absorptiometry scan and improves tissue structure by ultrasound in women with lipoedema and Dercum disease, Clin Obes, 8(2018):398-406.
[Crossref] [Google Scholar] [PubMed]
- M.J. Desai, R. Siriki, D. Wang, Treatment of pain in Dercum’s disease with Lidoderm (Lidocaine 5% patch): A case report, Pain Med, 9(2008):1224–1226.
[Crossref] [Google Scholar] [PubMed]
- A. Mosbeh, R. Almutairi, A. Albazzali, A. Albazali, Dercum's disease: A rare disease of painful fatty lumps, Cureus, 15(2023):e48615.
[Crossref] [Google Scholar] [PubMed]
- S. Martinenghi, A. Caretto, C. Losio, Successful treatment of Dercum’s disease by transcutaneous electrical stimulation: A case report, Medicine (Baltimore), 94(2015):e950.
[Crossref] [Google Scholar] [PubMed]
- Z. Gonciarz, W. Mazur, J. Hartleb, Interferon alfa-2b induced long-term relief of pain in two patients with adiposis dolorosa and chronic hepatitis C, J Hepatol, 27(1997):1141.
[Crossref] [Google Scholar] [PubMed]
- U. Lange, P. Oelzer, C. Uhlemann, Dercum’s disease (Lipomatosis dolorosa): Successful therapy with pregabalin and manual lymphatic drainage and a current overview, Rheumatol Int, 29(2008):17-22.
[Crossref] [Google Scholar] [PubMed]
- A. Singal, J.J. Janiga, N.M. Bossenbroek, H.W. Lim, Dercum’s disease (adiposis dolorosa): A report of improvement with infliximab and methotrexate, J Eur Acad Dermatol Venereol, 21(2007):717.
[Crossref] [Google Scholar] [PubMed]
- C. E. McKay, I. Batish, S. Arami, Infliximab-induced improvement in Dercum's disease, Cureus, 16(2024):e61499.
[Crossref] [Google Scholar] [PubMed]
- Wipf A, Lofgreen S, Miller DD, Farah RS, Novel use of deoxycholic acid for adiposis dolorosa (Dercum disease), Dermatol Surg, 45(2019):1718-1720.
[Crossref] [Google Scholar] [PubMed]
- A. H. Eliason, Y. I. Seo, D. Murphy, C. Beal, Adiposis dolorosa pain management, Fed Pract, 36(2019):529-533.
[Google Scholar] [PubMed]
- M.J. Desai, R. Siriki, D. Wang, Treatment of pain in Dercum’s disease with lidoderm (lidocaine 5% patch): A case report, Pain Med, 9(2008):1224-1226.
[Crossref] [Google Scholar] [PubMed]
- R.W. Loftus, M.P. Yeager, J.A. Clark, Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery, Anesthesiology, 113(2010):639-646.
[Crossref] [Google Scholar] [PubMed]
- S.P. Cohen, A. Bhatia, A. Buvanendran, Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American society of regional anesthesia and pain medicine, the American academy of pain medicine, and the American society of anesthesiologists, Reg Anesth Pain Med, 43(2018):521-546.
[Crossref] [Google Scholar] [PubMed]
- A.H. Eliason, Y.I.L. Seo, D. Murphy, C. Beal, Adiposis Dolorosa Pain Management, Fed Pract, 36(2019):529-533.
[Google Scholar] [PubMed]
- A. Caretto, E. Errichiello, M.G. Patricelli, O. Zuffardi, G. Cristel, et al. Transcutaneous electrical stimulation therapy and genetic analysis in Dercum's disease. A pilot study, Medicine (Baltimore), 100(2021):e28360.
[Crossref] [Google Scholar] [PubMed]
- S. Martinenghi, A. Caretto, C. Losio, M. Scavini, E. Bosi, Successful treatment of Dercum’s disease by transcutaneous electrical stimulation: A case report, Medicine (Baltimore), 94(2015):e950.
[Crossref] [Google Scholar] [PubMed]
- K.L. Herbst, T. Rutledge, Pilot study: Rapidly cycling hypobaric pressure improves pain after 5 days in adiposis dolorosa, J Pain Res, 3(2010):147–153.
[Crossref] [Google Scholar] [PubMed]
- E. Manheimer, K. Cheng, K. Linde, Acupuncture for peripheral joint osteoarthritis, Cochrane Database Syst Rev, 2010(2010):CD001977.
[Crossref] [Google Scholar] [PubMed]
- J.C. Deare, Z. Zheng, C.C. Xue, Acupuncture for treating fibromyalgia, Cochrane Database Syst Rev, 2013(2013):CD007070.
[Crossref] [Google Scholar] [PubMed]
- M.W.C. Chan, X.Y. Wu, J.C.Y. Wu, S.Y.S. Wong, V.C.H. Chung, Safety of acupuncture: Overview of systematic reviews, Sci Rep, 7(2017):3369.
[Crossref] [Google Scholar] [PubMed]
- Z. Gonciarz, W. Mazur, J. Hartleb, M. Machniak, I. Bednarek, et al. Interferon alfa-2b induced long-term relief of pain in two patients with adiposis dolorosa and chronic hepatitis C, J Hepatol, 27(1997):1141.
[Crossref] [Google Scholar] [PubMed]
- S.S. Greenbaum, J. Varga, Corticosteroid-induced juxta-articular adiposis dolorosa, Arch Dermatol, 127(1991):231-233.
[Google Scholar] [PubMed]
- R. Baron, Neuropathic pain: A clinical perspective, Handb Exp Pharmacol, 194(2009):3-30.
[Crossref] [Google Scholar] [PubMed]
- F. Beignon, M. Notais, S. Diochot, A. Baron, Z. Fajloun, Neurotoxins acting on TRPV1-building a molecular template for the study of pain and thermal dysfunctions, Toxins (Basel), 17(2025):64.
- B. Dong, D. Li, S. Song, N. He, S. Yue, et al. MTOR promotes astrocyte activation and participates in neuropathic pain through an upregulation of RIP3, Neurochem Res, 50(2025):93.
[Crossref] [Google Scholar] [PubMed]
- Z.J. Zhang, B.C. Jiang, Y.J. Gao, Chemokines in neuron-glial cell interaction and pathogenesis of neuropathic pain, Cell Mol Life Sci, 74(2017):3275-3291.
[Crossref] [Google Scholar] [PubMed]
- C. Sommer, M. Leinders, N. Uceyler, Inflammation in the pathophysiology of neuropathic pain, Pain, 159(2018):595-602.
[Crossref] [Google Scholar] [PubMed]
- R.R. Ji, C.R. Donnelly, M. Nedergaard, Astrocytes in chronic pain and itch, Nat Rev Neurosci, 20(2019):667-685.
[Crossref] [Google Scholar] [PubMed]
- Y. Fang, Astrocytic phosphatase and tensin homolog deleted on chromosome 10 regulates neuropathic pain by facilitating 3-hydroxy-3-methylglutaryl-CoA reductase-dependent cholesterol biosynthesis, Pain, 163(2022):e1192-e1206.
[Crossref] [Google Scholar] [PubMed]
- D.S. Lin, Rapamycin alleviates protein aggregates, reduces neuroinflammation, and rescues demyelination in globoid cell leukodystrophy, Cells, 12(2023):993.
[Crossref] [Google Scholar] [PubMed]
Copyright: © 2025 Lourdes de Fatima Ibanez Valdes, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.