Research Article: Journal of Drug and Alcohol Research (2022) Volume 11, Issue 9
Stability Indicative and Cost Effective Analytical Method Development and Validation Of Favipiravir and Oseltamivir in Bulk And Pharmaceutical Dosage Form By Using Rp-Hplc
A .Venkata Suresh Babu* and Hemant K.SharmaA .Venkata Suresh Babu, College of Pharmacy, Sri Satya Sai University of Technology and Medical Sciences, India, India, Email: suresh158@gmail.com
Received: 31-Aug-2022, Manuscript No. jdar-22-76609; Editor assigned: 02-Sep-2022, Pre QC No. jdar-22-76609 (PQ); Reviewed: 16-Sep-2022, QC No. jdar-22-76609; Revised: 21-Sep-2022, Manuscript No. jdar-22-76609 (R); Published: 28-Sep-2022, DOI: 10.4303/jdar/236196
Abstract
The current investigation was pointed at developing and progressively validating novel, simple, responsive and stable RP-HPLC method for the measurement of active pharmaceutical ingredients of Favipiravir and Oseltamivir and their related substances. A simple, selective, validated and well-defined stability that shows gradient RP-HPLC methodology for the quantitative determination of Favipiravir and Oseltamivir. The chromatographic strategy utilized Column of Dikma Inspire (4.6 mm × 250 mm, 5mm), using isocratic elution with a mobile phase of 0.1% orthophosphoric acid and Acetonitrile (83:37). A flow rate of 1 ml/min and a detector wavelength of 254 nm utilizing the 2487 UV detector were given in the instrumental settings. Using the impurity-spiked solution, the chromatographic approach was streamlined. Validation of the proposed method was carried out according to an international conference on harmonization (ICH) guidelines. LOD and LOQ for the two active ingredients and their impurities were established with respect to test concentration. The calibration charts plotted were linear with a regression coefficient of R2=0.999, which means the linearity was within the limit. Recovery, specificity, linearity, accuracy, robustness, ruggedness was determined as a part of method validation and the results were found to be within the acceptable range. The proposed method to be fast, simple, feasible and affordable in RS condition. During stability tests, it can be used for routine analysis of production samples and to verify the quality of drug samples during stability studies.
Keywords
Favipiravir; Oseltamivir; RP-HPLC; Development; Validation
Introduction
Oseltamivir is chemically (3R,4R,5S)-4-(Acetylamino)- 5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylic acid ethylester. It has an antiviral activity. Its active metabolite selectively blocks the viral surface enzyme neuraminidase thereby preventing the release of virus particles from infected cells. It is active against influenza A and B virus and is the drug of choice for treatment of swine flu. It comes under the category of drugs called neuraminidase inhibitors. It works by stopping the spread of flu virus in the body [1-4]. Oseltamivir phosphate (OSP) is the drug of choice for avian influenza caused by H1N1 virus. Due to rapid spread of pandemic influenza (swine flu) there may be chances of counterfeit products [5-9]. Favipiravir, sold under the brand name Avigan among others, is an antiviral medication used to treat influenza in Japan [10]. It is also being studied to treat a number of other viral infections [11]. There is evidence that use of during pregnancy may result in harm to the baby [12,13]. Research in 2014, suggested that Favipiravir may have efficacy in humans, it went unaddressed.
Materials and Methods
Chemicals
Acetonitrile, HPLC-grade Orthophosphoric acid, water, were purchased from Merck India Ltd, Mumbai, India. APIs of Favipiravir, Oseltamivir standards were procured from Honour Labs, Hyderabad.
The instrumentation
Waters alliance liquid chromatography (model 2695) monitored with empower 2.0 data handling system and a detector of UV (model 2487) was used for this study [14-17].
Method optimization
Initially the mobile phase tried was methanol: Ammonium acetate buffer and Methanol: Phosphate buffer with various combinations of pH as well as varying proportions. Finally, the mobile phase was optimized to orthophosphoric acid with buffer (pH 3.0), Acetonitrile in proportion 83:17 v/v respectively. UV spectrum of 10 μg/ml Favipiravir and Oseltamivir in diluents in methanol was recorded by scanning in the range of 200 nm to 400 nm. From the UV spectrum wavelength selected as 254 nm. At this wavelength both the drugs show good absorbance. UV spectrum of 10 μg/ ml Favipiravir and Oseltamivir in diluents in methanol was recorded by scanning in the range of 200 nm to 400 nm. From the UV spectrum wavelength selected as 254 nm. At this wavelength both the drugs show good absorbance. The developed HPLC method was utilized for the estimation of the combined drugs by in vitro method [18].
Validation procedure
The analytical parameters such as system suitability, precision, specificity, accuracy, linearity, robustness, LOD, LOQ, forced degradation and stability were validated according to ICH Q2 (R1) guidelines [19-28].
Preparation of buffer and mobile phase
Preparation of 0.1% OPA buffer: Pipette out 1 ml of Ortho Phosphoric Acid was taken in a 1000 ml volumetric flask, dissolved and diluted to 1000 ml with HPLC water and the volume was adjusted to pH 3.0 with triethylamine.
Preparation of mobile phase: Accurately measured 830 ml (83%) of above buffer and 170 ml of Acetonitrile HPLC (17%) were mixed and degassed in an ultrasonic water bath for 10 minutes and then filtered through 0.45 μ filter under vacuum filtration.
Diluent preparation: The Mobile phase was used as the diluent and showed in Figures 1-5.
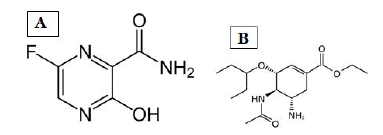
Figure 1: Structure A: Favipiravir B: Oseltamivir
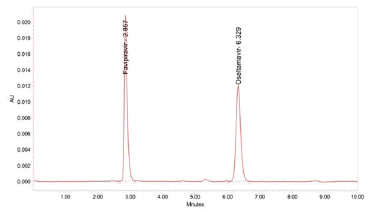
Figure 2: Chromatogram of system suitability
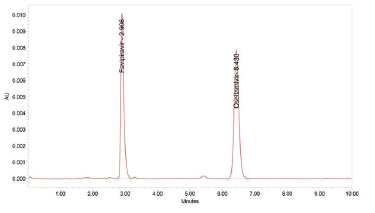
Figure 3: Chromatogram of Linearity
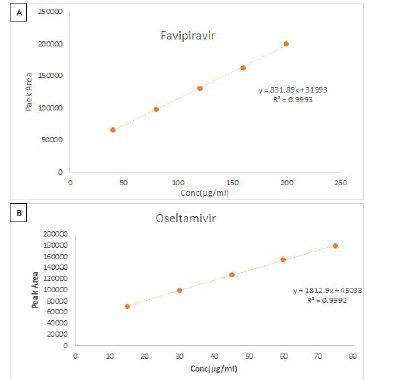
Figure 4: Calibration plots of (A) Favipiravir (B) Oseltamivir
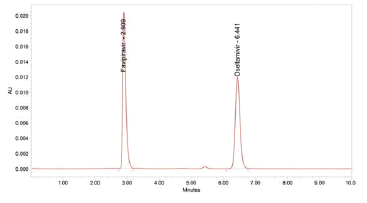
Figure 5: Chromatogram of Accuracy
Preparation of the Favipiravir and Oseltamivir standard and sample solution
Standard solution preparation: Accurately weigh and transfer 100 mg of Favipiravir and 37.5 mg of Oseltamivir working standards into a 25 ml clean dry volumetric flask add about 15 ml of Diluent and sonicate to dissolve it completely and make volume up to the mark with the same solvent (Stock solution).
Further pipette 0.3 ml of the above stock solutions into a 10 ml volumetric flask and dilute up to the mark with diluent.
Sample solution preparation
Accurately weigh 10 tablets of Favipiravir crush in mortar and pestle and transfer equivalent to 100 mg (i.e. 164 mg) of Favipiravir 25 ml clean dry volumetric flask add about 15 ml of Diluent and sonicate it up to 30 mints to dissolve it completely and make volume up to the mark with the same solvent. Then it is filtered through 0.44 micron Injection filter. (Stock solution of Favipiravir Sample)
Accurately weigh 10 Capsules of Oseltamivir powder crush in mortar and pestle and transfer equivalent to 37.5 mg of Oseltamivir (i.e. 79 mg) 25 ml clean dry volumetric flask add about 15 ml of Diluent and sonicate it up to 30 mints to dissolve it completely and make volume up to the mark with the same solvent. Then it is filtered through 0.44 micron Injection filter. (Stock solution of Oseltamivir Sample)
Further pipette 0.3 ml of each stock solutions of Favipiravir and Oseltamivir into a 10 ml volumetric flask and dilute up to the mark with diluent Figures 6-9.
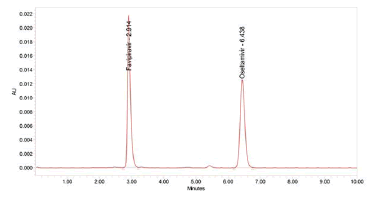
Figure 6: Chromatogram of Precision
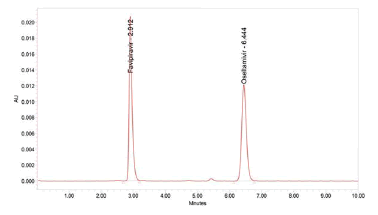
Figure 7: Chromatogram of Ruggedness
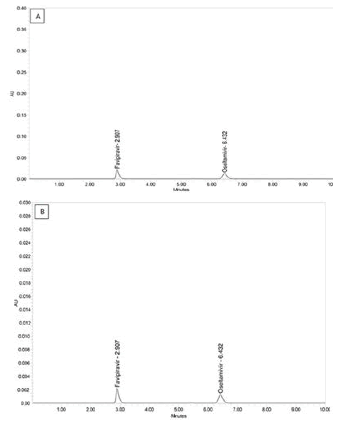
Figure 8: Chromatogram of (A) LOD and (B) LOQ
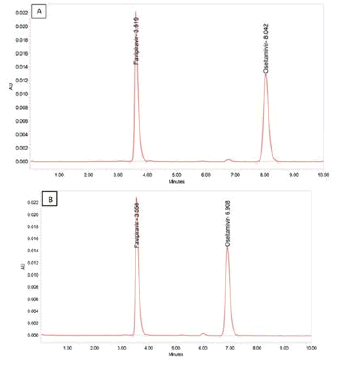
Figure 9: Chromatograms of (A&B) Robustness
Procedure
Inject 10 μL of the standard, sample into the chromatographic system and measure the areas for Favipiravir and Oseltamivir peaks and calculate the Assay% by using the formulae.
Results and Discussion
The main analytical challenge during the development of a new method was to separate active Pharma ingredients from their impurities. In order to provide a good performance, the chromatographic conditions were optimized.
System suitability
In System, suitability injecting standard solution and reported USP tailing and plate count values are tabulated in Table 1.
| System suitability Parameter | Acceptance criteria | Drug Name | |
|---|---|---|---|
| Favipiravir | Oseltamivir | ||
| USP Plate Count | NLT 2000 | 4509.57 | 9239.89 |
| USP Tailing | NMT 2 | 1.47 | 1.15 |
| USP Resolution | NLT 2 | - | 16.02 |
| %RSD | NMT 2 | 0.9 | 0.3 |
Table 1: Results of system suitability
Linearity
The area of the linearity peak versus different concentrations has been evaluated for Favipiravir, as 40, 80, 120, 160, 200 and Oseltamivir 15 μg/ml, 30 μg/ml, 45 μg/ml, 60 μg/ml, 75 μg/ml respectively. Linearity was performed in the range of 40 μg/ml-200 μg/ml of Favipiravir and 15 μg/ml-75 μg/ml of Oseltamivir. The correlation coefficients achieved 0.999 for all.
Accuracy
In this method, Accuracy was conducted in triplicate by analyzing active pharma ingredient sample solution spiked with known amounts of all the impurities at three kinds of concentration levels of 50%, 100% and 150% of each at a specified limit. For all impurities, percentage recoveries were measured and found to be within the limit. The accuracy and reliability of the developed method were established. The percentage recovery values were found to be in the range of 99%-102% for Favipiravir and Oseltamivir. The results are given in Tables 2 and 3.
| S. No | Linearity Level | Favipiravir | Oseltamivir | ||
|---|---|---|---|---|---|
| Concentration | Area | Concentration | Area | ||
| 1 | I | 40 | 65792 | 15 | 71267 |
| 2 | II | 80 | 98696 | 30 | 99725 |
| 3 | III | 120 | 131638 | 45 | 127369 |
| 4 | IV | 160 | 162911 | 60 | 155275 |
| 5 | V | 200 | 200063 | 75 | 179461 |
| Correlation Coefficient | 0.999 | 0.999 | |||
Table 2: Results of Linearity
| S. No. | % Level | Favipiravir % recovery | Oseltamivir % recovery |
|---|---|---|---|
| 1 | 50 | 100.02 | 100.37 |
| 2 | 100 | 100.67 | 100.87 |
| 3 | 150 | 100.9 | 99.16 |
| Mean | 100.53 | 100.13 |
Table 3: Results of Accuracy
Precision
The standard solution was injected for six times and measured the area for all six. Injections in HPLC. The RSD% for the area of six replicate injections was found to be within the specified limits.
Intermediate precision/ruggedness
To evaluate the intermediate precision (also known as Ruggedness) of the method, Precision was performed on different day. The standard solutions prepared in the precision were injected on the other day, for six times and measured the area for all six injections in HPLC. The RSD% for the area of six replicate injections was found to be within the specified limits Tables 4 and 5.
| Injection | Area for Favipiravir | Area for Oseltamivir |
|---|---|---|
| Injection-1 | 141368 | 128876 |
| Injection-2 | 140717 | 127224 |
| Injection-3 | 142655 | 129055 |
| Injection-4 | 143939 | 128739 |
| Injection-5 | 143013 | 126699 |
| Injection-6 | 142282 | 129220 |
| Average | 142329 | 128302.2 |
| Standard Deviation | 1156.8 | 1064.1 |
| %RSD | 0.8 | 0.8 |
Table 4: Results of Precesion
| Injection | Area for Favipiravir | Area for Oseltamivir |
|---|---|---|
| Injection-1 | 139453 | 122535 |
| Injection-2 | 137162 | 121224 |
| Injection-3 | 139458 | 122915 |
| Injection-4 | 138377 | 123391 |
| Injection-5 | 138482 | 123108 |
| Injection-6 | 139771 | 122959 |
| Average | 138783.8 | 122688.7 |
| Standard Deviation | 976.1 | 769.7 |
| %RSD | 0.7 | 0.6 |
Table 5: Results of Ruggedness
Robustness
The conditions of the experiment were designed to test the robustness of the established system intentionally altered, such as flow rate, mobile phase in organic percentage in all these varied conditions. Robustness results for Favipiravir and Oseltamivir found to be within the limit and results are tabulated in Tables 6-10.
| S.No | Peak Name | RT | Area | Height | % Area | USP Resolution | USP Tailing | USP Plate Count |
|---|---|---|---|---|---|---|---|---|
| 1 | Favipiravir | 2.907 | 1286.3 | 198 | 52.98 | - | 1.37 | 4553.46 |
| 2 | Oseltamivir | 6.432 | 1990.4 | 199 | 47.02 | 16.05 | 1.15 | 9283.78 |
Table 6: Results of LOD&LOQ
| Favipiravir | Oseltamivir | ||||||
|---|---|---|---|---|---|---|---|
| LOD | LOQ | LOD | LOQ | ||||
| Concentration | s/n | Concentration | s/n | Concentration | s/n | Concentration | s/n |
| 1.14µg/ml | 3 | 3.80 µg/ml | 10 | 0.74µg/ml | 3 | 2.46 µg/ml | 10 |
Table 7: Results of LOD&LOQ
| S.No | Flow Rate (ml/min) | System Suitability Results(Favipiravir) | System Suitability Results(Oseltamivir) | ||
|---|---|---|---|---|---|
| USP Plate Count | USP Tailing | USP Plate Count | USP Tailing | ||
| 1 | 0.9 | 4685.09 | 1.12 | 9731.46 | 1.21 |
| 2 | 1 | 4509.7 | 1.47 | 9509.7 | 1.47 |
| 3 | 1.1 | 4065.51 | 1.4 | 9549.3 | 1.12 |
Table 8: Results of LOD&LOQ
| S. No | Change in Organic Composition in the Mobile Phase | System Suitability Results (Favipiravir) | System Suitability Results (Oseltamivir) | ||
|---|---|---|---|---|---|
| USP Plate Count | USP Tailing | USP Plate Count | USP Tailing | ||
| 1 | 10% less | 4382.7 | 1.12 | 9643.64 | 1.26 |
| 2 | *Actual | 4509.7 | 1.47 | 4509.7 | 1.47 |
| 3 | 10% more | 4982.7 | 1.17 | 9287.28 | 0.95 |
Table 9: Results of Robustness
| Sample Name | Favipiravir | Oseltamivir | ||
|---|---|---|---|---|
| Area | % Degraded | Area | % Degraded | |
| Standard | 135383 | 100 | 121004.3 | 100 |
| Acid | 125453 | 7.33 | 115289 | 4.72 |
| Base | 127849 | 5.57 | 117420 | 2.96 |
| Peroxide | 125131 | 7.57 | 113076 | 6.55 |
| Thermal | 128347 | 5.2 | 113704 | 6.03 |
| Photo | 129359 | 4.45 | 116820 | 3.46 |
Table 10: Results of Degradation studies
Degradation studies
The International Conference on Harmonization (ICH) guideline entitled stability testing of new drug substances and products requires that stress testing be carried out to elucidate the inherent stability characteristics of the active substance. The aim of this work was to perform the stress degradation studies on the Favipiravir and Oseltamivir using the proposed method [29].
Preparation of stock
Accurately weigh and transfer 100 mg of Favipiravir and 37.5 mg of Oseltamivir working standard into a 25 ml clean dry volumetric flask add about 15 ml of Diluent and sonicate to dissolve it completely and make volume up to the mark with the same solvent. (Stock solution)
Further pipette 0.3 ml of the above stock solutions into a 10 ml volumetric flask and dilute up to the mark with diluent.
Hydrolytic degradation under acidic condition
Pipette 0.3 ml of above solution into a 10 ml volumetric flask and 3 ml of 0.1 N HCl was added. Then, the volumetric flask was kept at 60°C for 6 hours and then neutralized with 0.1 N NaOH and make up to 10 ml with diluent. Filter the solution with 0.22 microns syringe filters and place in vials.
Hydrolytic degradation under alkaline condition
Pipette 0.3 ml of above solution into a 10 ml volumetric and add 3 ml of 0.1 N NaOH was added in 10 ml of volumetric flask. Then, the volumetric flask was kept at 60°C for 6 hours and then neutralized with 0.1 N HCl and make up to 10 ml with diluent. Filter the solution with 0.22 microns syringe filters and place in vials.
Thermal induced degradation
Favipiravir and Oseltamivir sample was taken in petridish and kept in Hot air oven at 110°C for 24 hours. Then the sample was taken and diluted with diluents and injected into HPLC and analysed.
Oxidative degradation
Pipette 0.3 ml above stock solution into a 10 ml volumetric flask and 1 ml of 3% w/v of hydrogen peroxide added in 10 ml of volumetric flask and the volume was made up to the mark with diluent. The volumetric flask was then kept at room temperature for 15 minutes. Filter the solution with 0.45 microns syringe filters and place in vials.
Photo degradation
Pipette 0.3 ml above stock solution into a 10 ml volumetric flask and expose to sunlight for 24 hours and the volume was made up to the mark with diluent. Filter the solution with 0.45 microns syringe filters and place in vials.
Conclusion
We present in this article simple, selective, validated and well-defined stability that shows gradient RP-HPLC methodology for the quantitative determination of Favipiravir and Oseltamivir. All the products of degradation formed during the stress conditions and the related active pharma ingredients are well separated and peaks were well resolved from each other and separate with an appropriate retention time indicating that the proposed method to be fast, simple, feasible and affordable in RS condition.
Therefore the developed method during stability tests, it can be used for routine analysis of production samples and to verify the quality of drug samples during stability studies.
Acknowledgement
The authors thankful to management of Sri Satya Sai University of Technology and Medical Sciences for their support to complete this research work.
Conflict of Interest
No conflict of Interest.
Conclusion
No conflict of Interest.
References
- O. Neil, The merck index: An encyclopedia of chemicals, drugs and biologicals, merck and co. inc, 14(2006):1187-1188.
- S.C. Sweetman, The complete drug reference, pharmaceutical press, 140(2010):871–882.
[Crossref] [Google Scholar] [PubMed]
- J. Mckimm-Breschkin, Neuraminidase sequence analysis and susceptibilities of influenza virus clinical isolates to zanamivir and oseltamivir, Anti microb Agents chemother, 47(2003):2264-72.
[Crossref] [Google Scholar] [PubMed]
- D. Michael Green, H. Netty, A. Robert Wirtz, Rp-Hplc method development and validation for the estimation of oseltamivir phosphate in bulk form and pharmaceutical formulations, J Emg Inf Dis, 14(2008):4.
[Crossref] [Google Scholar] [PubMed]
- F. Jabbiribar, A. Mortazavi, R. Jalali-Milani, A. Jouyban, Analysis of oseltamivir in tamiflu capsules using micellarelectrokinetic chromatography, Chem Pharm Bull, 56(2008):1639-44.
[Crossref] [Google Scholar] [PubMed]
- H. Wiltshire, B. Wiltshire, A. Citron, T. Clarke, C. Serpe, et al. Determination of oseltamivir quality by colorimetric and liquid chromatographic methods, J Chromatgr B, 745(2000):373-88.
[Crossref] [Google Scholar] [PubMed]
- N. Lindergardh, Hien, J. Farrar, P. Singhasivanan, N.J. White, Rp-Hplc method development and validation for the estimation of oseltamivir phosphate in bulk form and pharmaceutical formulations, J Pharm Biomed Anal, 42(2006):430-433.
[Crossref] [Google Scholar] [PubMed]
- World Health Organisation. Fact sheet 275. Counterfeit medicines. Nov. 2006
- F. Hoffman–La Roche Ltd. Product monograph. Tamiflu
- Glenmark launches Covid-19 drug FabiFlu, priced at Rs 103 per tablet
- Y. X. Du, X. P. Chen, Favipiravir: Pharmacokinetics and concerns about clinical trials for 2019-nCov infection, Clin Pharmacol Ther, 108(2020):242-7.
[Crossref] [Google Scholar] [PubMed]
- K. Shiraki, T. Daikoku, Favipiravir, an anti-influenza drug against life-threatening RNA virus infections, Pharmacol Ther, 209(2020):107512.
[Crossref] [Google Scholar] [PubMed]
- S. Murugan, C. H. Upendra Janardhan, M. Niranjan Babu, RP-HPLC method for simultaneous estimation of albendazole and niclosamide in oral suspension for veterinary use, Res J Pharm Tech, 9(2016):27-32.
[Crossref] [Google Scholar] [PubMed]
- P. Bhavani, K. Prasada Rao, S. Mohan, Novel validated reversed-phase high-performance liquid chromatography method for determination of glucosamine, diacerein, and methyl sulfonyl methane in micro sample rat plasma and its application to pharmacokinetic and dissolution studies, Asian J Pharm Clin Res, 13(2020):50-63.
[Crossref] [Google Scholar] [PubMed]
- D. S. Naresh Kumar, D. Patel, Stability indicating chromatographic method development and va lidation for the simultaneous estimation of escitalopram oxalate and flupentixol in its pharmaceutical dosage form by HPLC, WJPR, 6(2017):549-66.
[Crossref] [Google Scholar] [PubMed]
- T. Supriya, D. Naresh, G. Vijaya Kumar, M. A. Haneer, Stability indicating RP-HPLC method developme nt and validation for simultaneous estimation of escitalopram and flupentixol pure and marketed formulation, Asian J Pharm Res, 8(2018):4-10.
[Crossref] [Google Scholar] [PubMed]
- C. P. Shivani, D. G. Maheshwari, Development and va lidation of UV spectrometric and HPLC method for estimation of escitalopram oxalate and flupentixol dihydrochloride in combined dosage form, AJPTI, 4(2016):59-70.
[Crossref] [Google Scholar] [PubMed]
- S. Malathi, D. Devakumar, Development and validation of Rp- Hplc method for the estimation of escitalopram oxalate and flupentixol dihydrochloride in combined dosage form and plasma, Int J Pharm Pharm Sci, 13(2021):61-6.
[Crossref] [Google Scholar] [PubMed]
- M. Naykode, A. Bhagwat, D. Jadhav, N. Harinath, Analytical and bio analytical method for quantification of pure azilsartan, not its salt by RP-HPLC, Res J Pharm Tech, 10(2017):708-14.
[Crossref] [Google Scholar] [PubMed]
- M. Singh, M. Charde, R. Shukla, M. C. Rita, Determination of calcipotriene in calcipotriene cream 0.05% w/w by RP-HPLC method development and validation, Res J Pharm Tech 4(2011):1219-23.
[Crossref] [Google Scholar] [PubMed]
- S. Malathi, N. Arunadevi, Development and validation of stability-indicating simultaneous estimation of metformin and alogliptin in tablets by high-performance thin layer chromatography, Int J Pharm Pharm Sci, 12(2020):68-73.
[Crossref] [Google Scholar] [PubMed]
- D. Senthil Rajan, G. Muruganathan, K. Shivkumar, T. Ganesh, Development and validation of hplc method for simultaneous quantification of vasicine, glycyrrhizin and piperine in poly herbal cough syrup, Int J Curr Pharm Res, 12(2020):15-9.
[Crossref] [Google Scholar] [PubMed]
- P. Shanmugasundaram, S. K. Kamarapu, RP-HPLC method for the simultaneous estimation and validation of amlodipine besylate and atenolol in bulk and tablet dosage form in biorelevant dissolution medium (Fassif), Res J Pharm Tech, 10(2017):3379-85.
[Crossref] [Google Scholar] [PubMed]
- S. Gomathy, S. T. Narenderan, S. N. Meyyanathan, B. Gowramma, Development and validation of hplc method for the simultaneous estimation of apigenin and luteolin in commercial formulation, J Crit Rev, 7(2020):4785-90.
[Crossref] [Google Scholar] [PubMed]
- S. Ashutosh Kumar, M. Debnath, J.V.L.N. Seshagiri, D. Gowri Sankar, Development and validation of a sensitive RP-HPLC method for simultaneous estimation of rosuvastatin and fenofibrate in tablet dosage form by using PDA detector in gradient mode, Res J Pharm Tech, 9(2016):549-54.
[Crossref] [Google Scholar] [PubMed]
- Y. Malak, A. A. Al-Bathish, Gazy MK, El-Jamal, Rp-hplc and chemometric methods for the determination of two anti-diabetic mixtures; Metformin hydrochloride-canagliflozin and metformin hydrochloride-gliclazide in their pharmaceutical formulation, Int J Pharm Pharm Sci, 12(2020):83-94.
[Crossref] [Google Scholar] [PubMed]
- M. P. Gadhvi, A. Bhandari, B. N. Suhagia, U. H. Desai, Development and validation of RP-HPLC method for simultaneous estimation of atazanavir and ritonavir in their combined tablet dosage form, Res J Pharm Tech, 6(2013):200-3.
[Crossref] [Google Scholar] [PubMed]
- K. Swati, P. Abhishek, S. Sushank, C. Bothiraja, P. Atmaram, High-performance liquid chromatography for the simultaneous estimation of cefoperazone and sulbactam in rat plasma and its importance in therapeutic drug monitoring, Int J Pharm Pharm Sci, 12(2020):92-7.
[Crossref] [Google Scholar] [PubMed]
- M. Vijayakumari, C. H. Balasekhar Reddy, Stability indicating validated hplc method for the determination of zanubrutinib in bulk and pharmaceutical dosage form, Asian J Pharm Clin Res, 13(2020):159-62.
[Crossref] [Google Scholar] [PubMed]
Copyright: © 2022 A Anbassa. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

