Research Article: Journal of Drug and Alcohol Research (2025) Volume 14, Issue 11
The antifungal activity prediction and the toxicity of the main phytoconstituents of an apiaceous species from Algeria
Elkolli Meriem1*, Elkolli Hayet2 and Bouzid Djihane12Department of Microbiology, Laboratory of Multiphasic Polymeric Materials, Départment of Process Eng, Faculty of Technology, University Ferhat Abbas of Setif 1, Setif 19000, Algeria
Elkolli Meriem, 1Department of Microbiology, Laboratory of Applied Microbiology, Faculty of Natural and Life Science, University of Ferhat Abbas Setif 1, Setif 19000, Algeria, Email: elkollim@yahoo.fr
Received: 02-Jun-2025, Manuscript No. JDAR-25-140960; Editor assigned: 04-Jul-2025, Pre QC No. JDAR-25-140960 (PQ); Reviewed: 18-Jul-2025, QC No. JDAR-25-140960; Revised: 18-Jul-2025, Manuscript No. JDAR-25-140960 (R); Published: 04-Aug-2025, DOI: 10.4303/JDAR/236482
Abstract
The aim of this work was to determine the constituent(s) responsible for the pre-established in vitro antifungal activity of the whole essential oil of the Apiaceous species Daucus crinitus. To this end, eight fungal proteins, with different roles in the cell, were targeted (2QZX, 1BWK, 5FRB, 6K3H, 1EAG, 5UIV, 7WJM and 8JZN) by the major compounds of the essential oil. To investigate how the selected phytocompounds interact with the enzym’s active sites, we employed a molecular docking study using Autodock Vina integrated into PyRx. Molinspiration Cheminformatics free web services and SwissADME free online tools were used to predict physicochemical and pharmacokinetic parameters, while OSIRIS Property Explorer online tools were used to predict toxicity risks. The β-caryophellene recorded the best energies with three enzymes; 5FRB, 8JZN and 7WJM with -8.6, -7.6 and -7.4 kcal/mol respectively, followed by Germacrene D, Isochavicol isobutyrate and β-sesquiphellandrène with binding energies sometimes close to those of the natural ligands of enzymes. The isochavicol isobutyrate, which is the major compound of the oil (39%), showed excellent pharmacokinetic properties without toxicity. This study suggests that the essential oil constituents could enhance the efficacy of antifungal drugs with the potential benefit of reducing the use of the latter in order to limit or slow down antibiotic resistance.
Keywords
Daucus crinitus; Antifungal; Molecular docking; Toxicity prediction
Introduction
Algeria is the largest country in Africa by land area and the tenth largest in the world, and is therefore rich in plant biodiversity with vast resource of medicinal plants. Local populations benefit from this biodiversity in herbal medicine to treat various infectious diseases because it is safe, inexpensive and widely accessible. In recent decades, even the industrialized world has seen an increase in the use of complementary and alternative medicine [1]. Among plant-derived products, Essential Oils (Eos) possess potent and broad-spectrum antimicrobial activities [2]. The family Apiaceae is characterized by releasing a relative high amount of EOs [3].
On the other hand, fungal infections cause 11.5 million serious cases and 1.5 million deaths worldwide each year [4]. Essential oils extracted from Apiaceous plants exhibit a wide range of effects, including antimicrobial and antibiotic effects [5], which corroborates the results of in vitro tests found subsequently on some fungi and yeasts [6]. This work aims to determine the composition/activity relationship of the essential oil of the aerial parts of the species D. crinitus, followed by the prediction of the pharmacotoxicity of its essential compounds.
Materials and Methods
After finding that D. crinitus essential oil exerts in vitro activity on fungi and yeasts (Table 1), we continued the molecular docking to understand the relationship between the oil composition and the antifungal effect at the molecular level.
| Fungal strain Origin | Concentration (Essential oil in DMSO) Inhibition zone in mm | |||
|---|---|---|---|---|
| 1/2 v/v | 1/5 v/v | 1/10 v/v | ||
| Aspergillus niger | Contaminant | 44 | 30 | 20 |
| Aspergillus fumugatus |
Contaminant | 12 | 10 | 8 |
| Penicillium notatum |
/ | 20 +/- | 20 +/- | 20 +/- |
| Fusarium rosum | / | 12 | 10 | 8 |
| Trychosporum sp | Vaginal swab | 33 | 28 | 21 |
| Candida albicans | Skin | 30 +/- | 25 +/- | 20 +/- |
Note: +/-: Less dense area
Table 1: The antifungal activity of D. crinitus essential oil.
Ligand and targets choice
For the study of molecular docking as well as for pharmacotoxicity, the main compounds (>2%) were selected and their 3d structures were used (Table 2).
| Compound | % | Pubchem CID |
|---|---|---|
| Isochavicol isobutyrate | 39 | 16729190 |
| Octyl-acetate | 12,3 | 8164 |
| α-pinene | 9,9 | 6654 |
| β-caryophellene | 5,4 | 5281515 |
| Myrcene | 3,4 | 31253 |
| β-farnesene | 3,4 | 5281517 |
| n-pentadecane | 2,8 | 12391 |
| β-sesquiphellandrene | 2,6 | 12315492 |
| Germacrene D | 2,3 | 5317570 |
Table 2: The selected phytoconstituents for the in silico study.
Protein targets were selected according to the literature (Table 3). Eight vital proteins with diverse roles in the fungal cell were the subject of the in silico study.
| Protein | Role | Organism source | PDB code | Natural ligand/CID |
|---|---|---|---|---|
| Aspartyl proteases [7] | Involved in the degradation of host proteins, thus facilitating tissue invasion | C. albicans | 2QZX | Pepstatin/5478883 |
| Old Yellow Enzyme (OYE1) [8] | Involved in resistance to oxidative stress and adaptation to hostile conditions | C. albicans | 1BWK | Flavin Mononucleotide/643976 |
| 14-α demethylase [9] | Essential in the biosynthesis of sterols playing a key role in the formation of ergosterol | A. fumigatus | 5FRB | (R)-4-((4-((6-(2-(2,4-difluorophenyl)-1,1-difluoro-2-hydroxy-3-(1H-tetrazol-1-yl)propyl)pyridin-3-yl)ethynyl)phenoxy)methyl)benzonitrile/139592435 |
| Nucleoside diphosphokinase [9] | Key enzyme in the energy metabolism of fungal cells | A. flavus | 6K3H | Adenosine-5'-diphosphate/6022 |
| Aspartic proteinase (SAP2) [10] | Hydrolyzes peptide bonds of host proteins | C. albicans | 1EAG | N-ethyl-N-[(4-methylpiperazin-1-yl)carbonyl]-D-phenylalanyl-N-[(1S,2S,4R)-4-(butylcarbamoyl)-1-(cyclohexylmethyl)-2-hydroxy-5-methylhexyl]-L-norleucinamide/5496932 |
| Thymidylate Kinase [11] | Catalyzes the phosphorylation of thymidine monophosphate (dTMP) to thymidine diphosphate (dTDP), a key step in DNA synthesis | C. albicans | 5UIV | dTMP/9700 |
| Chitin synthase [12] | Chitin biosynthesis | Phytophthora sojae | 7WJM | UDP-GlcNAc/445675 |
| Fungal 1,3-glucan synthase [12] | Synthesis of the fungal cell wall | S. cerevisiae | 8JZN | UDP-Glucose/8629 |
Table 3: The targeted proteins for the antifungal study.
In silico antifungal study
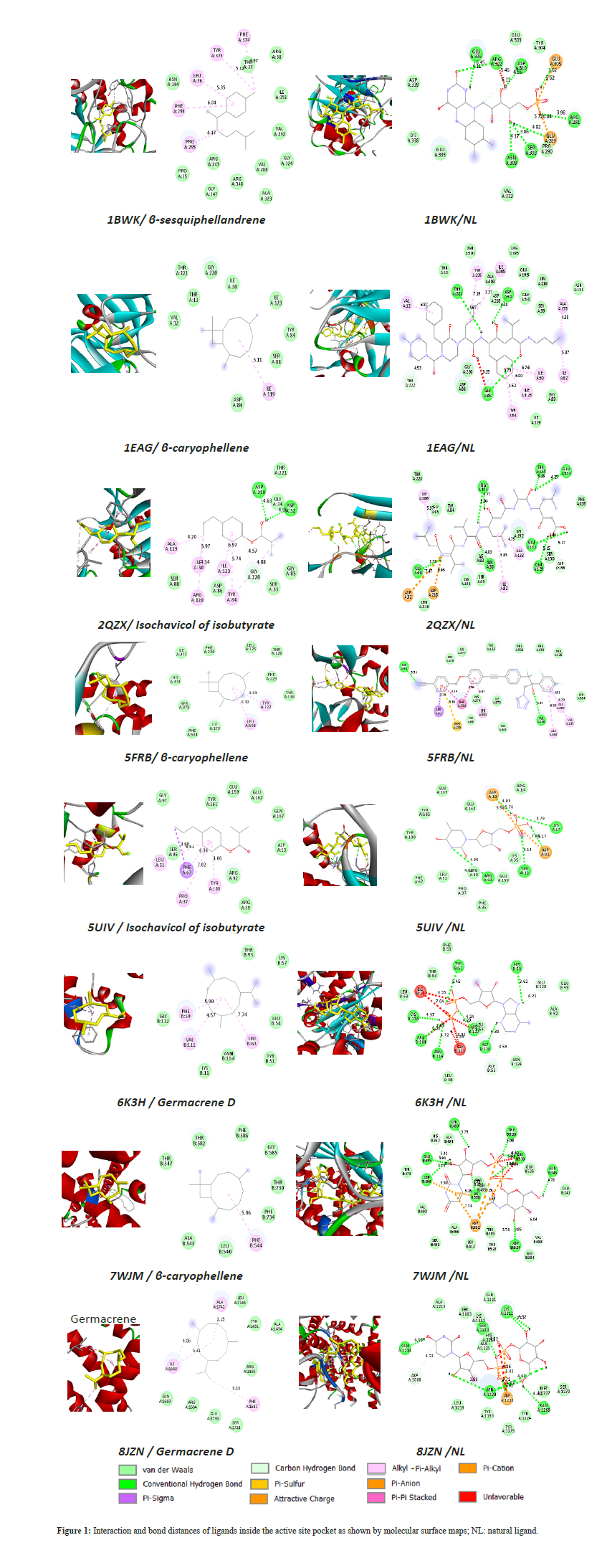
The combined in vitro and in silico approach allows us to better understand the link between the chemical composition of the oil and its biological activity. For this, molecular docking of the phytocompounds as well as the natural ligands was done against the selected targets proteins with PDB IDs: 2QZX, 1BWK, 5FRB, 6K3H, 1EAG, 5UIV, 7WJM and 8JZN to determine their binding affinities. The 3d structures of the phytocompounds and ligands were obtained in SDF format files from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) and Molview (https://molview.org/?cid=5478883) then converted to PDB format by Open Babel tools version 2.4.1. The proteins energies optimization was realized using Chem3D v 16.0.1.4 software. The 3d structures of the target proteins were obtained in PDB format from the Protein Data Bank (https://www.rcsb.org/). Autodock Vina in PyRx 0.8 was used to generate the best predicted binding modes and corresponding binding energies. The interactions were visualized in 3d and 2d forms using BIOVIA Discovery Studio visualizer 2024 v.24.1.0.23298 software. Bonds between the ligands and interacting residues are depicted with a distance range of Å.
Pharmacokinetics and toxicity prediction
Lipinski’s method was employed to assess the drug-like properties of phytocompounds, which sets limits on four specific physicochemical parameters [13]. These are the characteristics of an orally active drug: the octanol-water partition coefficient (milogP) and the number of hydrogen bond donors (n-OH and n-NH) should not exceed 5, and the number of hydrogen bond acceptors (n-ONs) should be less than 10. The molecular weight (MW) should be below 500 D, and no more than one violation should occur [14]. Molinspiration Cheminformatics free web services (https://www.molinspiration.com) and SwissADME free online tools (http://www.swissadme.ch/) were used to predict physicochemical and pharmacokinetic parameters, while OSIRIS Property Explorer online tools (https://www.organic-chemistry.org/prog/peo/) were used to predict toxicity risks.
Results and Discussion
Extraction and isolation of bioactive compounds from traditional medicine could be an interesting strategy for the discovery of new drugs [15]. Recently, computer-aided drug discovery approaches have attracted increasing attention as they can help alleviate the scale, time, and cost issues faced by conventional experimental approaches [16]. This process is typically accomplished by first predicting the molecular orientation of a ligand in a receptor and then estimating their complementarity through the use of a scoring function [17]. The results of this study show that all phytocompounds fit in the active site of the selected targets with binding energies ranging from −4.2 to −8.6 kcal/mol (Table 4). Molecular docking results reveal that the combinations of fungal protein targets 2QZX and 5UIV with Isochavicol isobutyrate, the main component of the oil, exhibit favorable binding energies (−6.1 and −7.0 kcal/mol respectively), higher than those of natural ligands, suggesting good inhibitory potential. Similarly, β-caryophyllene stood out for its ability to effectively interact with several key targets of C. albicans, including 1EAG, 5FRB, 8JZN, and 7WJM (−8.6, −7.6 and −7.4 kcal/mol respectively). Germacrene D also showed notable affinity with 5FRB and 8JZN. The biological implication of the targets studied, including 2QZX in biofilm formation [18] and infection [19], 5UIV in fungal growth [20], 5FRB in maintaining the integrity and function of cell membranes [21], 1EAG in the adhesion and invasion [22] and 8JZN in cell wall biosynthesis [23], reinforces the relevance of these interactions. These results suggest a promising therapeutic potential of these natural compounds, particularly as antifungal agents targeting key mechanisms of C. albicans.
| Ligand/Protein | β-caryophellene | n-pentadecane | β-farnesene | Germacrene D | Isochavicol isobutyrate | Myrcene | Octylacetate | α-pinene | β-sesquiphellandrene | Natural ligand |
|---|---|---|---|---|---|---|---|---|---|---|
| Binding energies (kcal/mol) | 7WJM | 7WJM | 7WJM | 7WJM | 7WJM | 7WJM | 7WJM | 7WJM | 7WJM | 7WJM |
| 1BWK | -5.9 | -5.1 | -4.6 | -6.5 | -5.3 | -4.6 | -5 | -5.5 | -6.6 | -6.8 |
| 1EAG | -6.5 | -4.6 | -4.7 | -6.2 | -5.9 | -4.6 | -4.4 | -5.1 | -5.6 | -7.8 |
| 2QZX | -5.7 | -4.6 | -5.3 | -5.5 | -6.1 | -4.9 | 4.2 | -5.1 | -5.8 | -5.8 |
| 5FRB | -8.6 | -5.9 | -6.3 | -7.8 | -6.6 | -5.1 | -5.1 | -6.4 | -6.8 | -11.4 |
| 5UIV | -6.6 | -5.8 | -6.4 | -5.6 | -7 | -6.2 | -5.8 | -5.8 | -6 | -5.9 |
| 6K3H | -6 | -4.4 | -4.9 | -6.1 | -5.9 | -4.3 | -4.5 | -4.7 | -5.8 | -8.1 |
| 7WJM | -7.4 | -4.6 | -5 | -6.8 | -6.2 | -5.4 | -4.2 | -6 | -5.5 | -8.7 |
| 8JZN | -7.6 | -5.2 | -5.6 | -7.8 | -6.2 | -5.3 | -5.2 | -6.4 | -7 | -8.3 |
Table 4: Phytocompounds scoring results.
The essential oils of different species of the Apiaceae family proved to be a promising source of biomolecules, with antifungal potential activity [3]. The relative concentration of the principal compounds determines the biological properties of EOs [24]. Therefore, by linking these results with those in vitro, isochavichol isobutyrate could be involved in less dense areas in C. albicans and Trychosporum sp. colonies, probably by interfering with fungal DNA synthesis, knowing that C. albicans is the most common cause of fungal infections in a growing population of immune-compromised patients [15].
On the other hand, Dahham et al. (2015) reported a significant activity of β-caryophyllene against A. niger, P. citrinum, R. oryzae and T. reesei; this activity could be attributed to its strong antioxidant activities. According to [25], Germacrene D was listed among chemical compounds with potential antimicrobial activity against C. albicans that were present in at least 50% of the 33 essential oils studied [25]. However, it is known that in a plant extract rich in phytocompounds, these compounds can act either individually, synergistically or antagonistically. At the molecular level, it was found that the majority of bonds involved in the active sites by the ligands are alkyl and VDW bonds, whereas with natural ligands there are many hydrogen bonds which give rise to better energies and contribute to the stabilization of protein–ligand complexes. Isochavicol isobutyrate interacts with GLU162, TYR161 and ARG92, LEU51, PHE67 and PRO37 like the natural ligand, which explains a good binding energy but slightly reduced stability (Figure 1).
Figure 1: Histology of the liver: Control group at X400-Section
Current first-line antifungal agents such as Amphotericin B, Fluconazole and Itraconazole may decrease the severity of fungal infection to some extent, but poor drug bioavailability, drug toxicity and poor water solubility seriously restrict their clinical utility [26]. Concerns regarding the safety of synthetic compounds have encouraged more detailed studies of plant resources [27]. For this essential oil, the major compound isochavicol isobutyrate is the safest according to in silico prediction, exhibiting good solubility and absorption, making it suitable for oral or cutaneous administration (Table 5). Most compounds have miLogP values below 5, except β-caryophyllene (5.17), β-farnesene (5.84), n-pentadecane (8.19), and Germacrene D (5.43), indicating low aqueous solubility. Increased lipophilicity may lead to accumulation in fatty tissues and prolonged presence in the body, influencing pharmacological activity and toxicity [28]. The low TPSA values suggest good membrane permeability, and TPSA values below 140 are considered ideal for drug-like molecules [29]. All compounds show good skin permeability coefficients (log Kp) ranging from −4.93 to −2.10 cm/s, within the acceptable range of −8.0 to −1.0 cm/s [30]. Cytochromes (CYP) are key enzymes involved in drug metabolism [31], particularly CYP2C9, CYP2C19, CYP2D6 and CYP3A4 [32]. β-farnesene and n-pentadecane inhibit CYP1A2 which may slow down the degradation of some drugs, thus increasing their plasma concentration and potentially their side effects thus influencing their toxicity. It is one of the most important enzymes in the liver which metabolises many clinical drugs [33].
| Compound | Isochavicol isobutyrate | Octyl-acetate | α-pinene | β-caryophellene | Myrcene | β-farnesène | n-pentadecane | β-sesquiphellandrene | GermacreneD |
|---|---|---|---|---|---|---|---|---|---|
| Physicochemical and pharmacokinetic parameters (Molinspiration Cheminformatics) | < | 3.5 | 3.84 | 3.54 | 5.17 | 3.99 | 5.84 | 8.19 | 4.9 |
| TPSA (oA)<500 | 26.3 | 26.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MW<500 (g/mol) | 204.27 | 172.27 | 136.24 | 204.36 | 136.24 | 204.36 | 212.42 | 204.36 | 204.36 |
| MV | 205.94 | 191.34 | 151.81 | 229.95 | 162.24 | 239.82 | 264.18 | 234.9 | 234.9 |
| nON<10 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| nOHNH<5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lipinski’s violation | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 |
| Solubility and pharmacokinetics properties (SwissADME) | |||||||||
| Water solubility | S | S | MS | MS | S | MS | PM | MS | MS |
| BBB permeant | Yes | Yes | Yes | No | Yes | No | No | No | No |
| Gastrointestinal absorption | High | High | Low | Low | Low | Low | Low | Low | Low |
| Log Kp : Skin permeation: cm/s | -4.9 | -4.93 | -3.95 | -4.44 | -4.17 | -3.27 | -2.1 | -3.71 | -4.18 |
| Cytochromes inhibitors | CYP1A2 | No | No | No | No | No | Yes | Yes | No |
| CYP2C19 | No | No | No | Yes | No | No | No | Yes | No |
| CYP2C9 | No | No | Yes | Yes | No | Yes | No | Yes | Yes |
| CYP2D6 | No | No | No | No | No | No | No | No | No |
| CYP3A4 | No | Yes | No | No | No | No | No | No | No |
| Toxicity risks (OSIRIS Property Explorer) | -7.8 | -7.8 | -7.8 | -7.8 | -7.8 | -7.8 | -7.8 | -7.8 | -7.8 |
| Mutagenic | No | No | No | No | No | No | No | No | No |
| Tumorigenic | No | No | No | No | Yes | MR | No | No | No |
| Irritant | No | Yes | Yes | No | Yes | MR | No | Yes | No |
| Reproductive effective | No | No | No | No | Yes | MR | No | No | No |
Note: miLogP: Logarithm of partition coefficient between n-octanol and water. TPSA: Topological polar surface area. MW: Molecular weight. MV: Molecular volume. nON: Number of hydrogen bond acceptors. nOHNH: Number of hydrogen bond donors. No: no indication found. S: Soluble. MS: Moderate to soluble. PM: poor to moderate. VS: very soluble, MR medium risk
Table 5: Calculated physicochemical and pharmacokinetic parameters of the docked phyto compounds
CYP2C19 is inhibited by β-caryophellene and β-sesquiphellandrene; it is also responsible for the metabolism of approximately 10% of commonly used therapeutic agents [34]. CYP2C9, which constitutes approximately 20% of the cytochrome P450 protein content of human liver microsomes [35], is inhibited by α-pinene, β-caryophellene, β-farnesene, β-sesquiphellandrene and Germacrene D. Octyl-acetate inhibits CYP3A4, which is implicated in the metabolism of drugs. Such unwanted inhibitions may have clinical consequences ranging from lack of therapeutic efficacy to severe toxicity. Only CYP2D6 is not inhibited by any molecule. Importantly, although CYP2D6 constitutes just 2%–4% of total hepatic CYP content, it is a cardinal drug-metabolising enzyme involved in the metabolism of approximately 20% of commonly used drugs [36]. Finally, some compounds present moderate irritant risks (Octyl-acetate and α-pinene), while myrcène shows tumorigenic, irritant and reproductive risks, which may exclude it from use as a potential drug. Indeed, despite the therapeutic benefits observed, β-myrcene has come under scientific scrutiny due to an alleged risk as a potential human carcinogen [37].
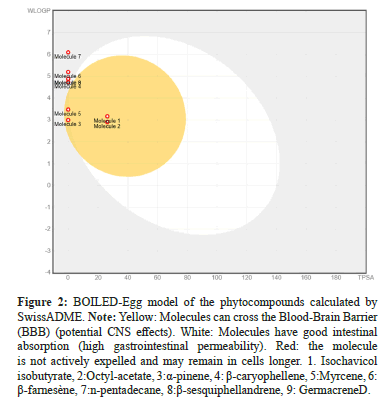
BOILED-Egg is an intuitive method to predict simultaneously two key ADME parameters, i.e. the passive gastrointestinal absorption and Brain access (BBB). Although conceptually very simple as it relies on two physicochemical descriptors only (WLOGP and TPSA, for lipophilicity and apparent polarity [31]. The Figure 2 represents the localisation of our phytoconstituents; points located in the yellow region are molecules predicted to passively permeate through the BBB, while points located in the white region are molecules predicted to be passively absorbed by the gastrointestinal tract. Red dots are for molecules predicted to not to be effluated from the central nervous system by the P-glycoprotein [38]. Molecules 1, 2, 3 and 5 are located in the yellow zone, meaning they can potentially cross the Blood-Brain Barrier (BBB) and exert an effect on the central nervous system. Molecules 4,6 and 8 are located outside the yellow zone, in the white zone, meaning they have good gastrointestinal absorption but are unlikely to cross the BBB. Molecule 7, has a very high WLOGP (8.19), indicating excessive lipophilicity, which may be a problem for solubility and bioavailability.
Figure 2: BOILED-Egg model of the phytocompounds calculated by SwissADME. Note: Yellow: Molecules can cross the Blood-Brain Barrier (BBB) (potential CNS effects). White: Molecules have good intestinal absorption (high gastrointestinal permeability). Red: the molecule is not actively expelled and may remain in cells longer. 1. Isochavicol isobutyrate, 2:Octyl-acetate, 3:α-pinene, 4: β-caryophellene, 5:Myrcene, 6: β-farnesène, 7:n-pentadecane, 8:β-sesquiphellandrene, 9: GermacreneD.
Conclusion
In this study, we presented the antifungal and pharmacokinetic profile, drug similarity, and toxicity profile of nine main compounds from D. crinitus essential oil, which demonstrated high binding affinity to fungal target proteins and favorable pharmacokinetic properties. Molecular docking results suggest that β-caryophellene, germacrene D, and β-sesquiphellandrene also contribute to the antifungal effect suggesting a promising therapeutic potential of these natural compounds, particularly as antifungal agents targeting key mechanisms of C. albicans. Moreover, in silico toxicity predictions indicate that isochavicol isobutyrate is a promising candidate for further development due to its low toxicity and good bioavailability. These results suggest that D. crinitus essential oil constituents could serve as potential antifungal agents or enhancers of existing antifungal treatments, potentially reducing the need for synthetic antifungal drugs and mitigating resistance. Further experimental validation is needed to confirm these in silico predictions and explore their clinical applicability.
References
- H.â¯Hemmami, B.B.â¯Seghir, S.â¯Zeghoud, I.â¯Benâ¯Amor, I.â¯Kouadri, etâ¯al. Desert endemic plants in Algeria: A review on traditional uses, phytochemistry, polyphenolic compounds and pharmacological activities. Mol,â¯28 (2023):â¯1834. [Crossref] [Google Scholar] [PubMed]
- A.â¯Trifan, S.V.â¯Luca, A.C.â¯BostÄnaru, M.â¯Brebu, A.â¯JitÄreanu, etâ¯al. Apiaceae essential oils: Boosters of terbinafine activity against dermatophytes and potent antiâinflammatory effectors. Plants,â¯10 (2021):â¯2378. [Crossref] [Google Scholar] [PubMed]
- J.N.â¯Vieira, C.L.â¯Gonçalves, J.P.V.â¯Villarreal, V.M.â¯Gonçalves, R.G.â¯Lund, etâ¯al. Chemical composition of essential oils from the Apiaceae family, cytotoxicity, and their antifungal activity in vitro against Candida species from oral cavity. Braz J Biol,â¯79 (2019):â¯432–437. [Crossref] [Google Scholar] [PubMed]
- K.V.â¯Naveen, K.â¯Saravanakumar, A.â¯Sathiyaseelan, D.â¯MubarakAli, M.H.â¯Wang, etâ¯al. Human fungal infection, immune response, and clinical challenge—a perspective during COVIDâ19 pandemic. Appl Biochem Biotechnol,â¯194 (2022):â¯4244–4257. [Crossref] [Google Scholar] [PubMed]
- A.â¯Khemili, D.â¯Bensizerara, H.â¯Chenchouni, R.â¯Chaibi, N.â¯Aissani, etâ¯al. Biological potential and essential oil profile of two wild Apiaceae species from Algeria (Daucus carota L. and Foeniculum vulgare Mill.): Larvicidal and antibacterial effects. Mol.,â¯29 (2024):â¯4614. [Crossref] [Google Scholar] [PubMed]
- M.â¯Elkolli. Contribution à l’étude de la composition chimique et de l’activité antimicrobienne des huiles essentielles d’Anthemis pedunculata Desp., d’Anthemis punctata Vahl. et de Daucus crinitus Desf. Thesis.â¯(2008). [Google Scholar]
- M.I.â¯Sulistyowaty, G.S.â¯Putra, T.â¯Budiati, A.W.â¯Indrianingsih, F.â¯Anwari, etâ¯al. Synthesis, in silico study, antibacterial and antifungal activities of Nâphenylbenzamides. Int J Mol Sci,â¯24 (2023):â¯2745. [Crossref] [Google Scholar] [PubMed]
- D.â¯Ríos, J.A.â¯Valderrama, G.â¯Quiroga, J.â¯Michea, F.â¯Salas, etâ¯al. Antifungal activity and in silico studies on 2âacylated benzoâ and naphthohydroquinones. Mol.,â¯27 (2022):â¯3035. [Crossref] [Google Scholar] [PubMed]
- B.â¯Khanzada, N.â¯Akhtar, M.K.â¯Okla, S.A.â¯Alamri, A.â¯AlâHashimi, etâ¯al. Profiling of antifungal activities and in silico studies of natural polyphenols from some plants. Mol,â¯26 (2021):â¯7164. [Crossref] [Google Scholar] [PubMed]
- A.â¯Jibrinâ¯Uttu, M.â¯Saniâ¯Sallau, H.â¯Ibrahim, I.O.â¯Risikatâ¯Agbeke, etâ¯al. In silico modelling and NMR characterization of some steroids from Strychnos innocua (Delile) root bark as potential antifungal agents. Steroids,â¯194 (2023):â¯109222. [Crossref] [Google Scholar] [PubMed]
- D. Fernandez, A. Restrepo-Acevedo, C. Rocha-Roa, R. Le Lagadec, R. Abonia, et al. Synthesis, structural characterization, and in vitro and in silico antifungal evaluation of azo-azomethine pyrazoles (PhN2(PhOH)CHN (C3N2(CH3)3)PhR, R = H or NO2). Mol., 26 (2021): 7435. [Crossref] [Google Scholar] [PubMed]
- R. Vibha, D.L. Granada, S.P.U.k.S. Skariyachan. In vitro and in silico investigation deciphering novel antifungal activity of endophyte Bacillus velezensis CBMB205 against Fusarium oxysporum. Sci Rep, 15 (2025): 684. [Crossref] [Google Scholar] [PubMed]
- C.A. Lipinski. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol, 1 (2004): 337–341. [Crossref] [Google Scholar] [PubMed]
- L. Adjissi, N. Chafai, K. Benbouguerra, I. Kirouani, A. Hellal, et al. Synthesis, characterization, DFT, antioxidant, antibacterial, pharmacokinetics and inhibition of SARS-CoV-2 main protease of some heterocyclic hydrazones. J Mol Struct, 1270 (2022): 134005. [Crossref] [Google Scholar] [PubMed]
- Q. Liu, W. Luyten, K. Pellens, Y. Wang, W. Wang, et al. Antifungal activity in plants from Chinese traditional and folk medicine. J Ethnopharmacol, 143 (2012): 772–778. [Crossref] [Google Scholar] [PubMed]
- B. Shaker, S. Ahmad, J. Lee, C. Jung, D. Na, et al. In silico methods and tools for drug discovery. Comput Biol Med, 137 (2021): 104851. [Crossref] [Google Scholar] [PubMed]
- L. Pinzi, G. Rastelli. Molecular docking: shifting paradigms in drug discovery. Int J Mol Sci, 20 (2019): 4331. [Crossref] [Google Scholar] [PubMed]
- S. Yan, J.A.H. Kowah, Q. Long, Q. Liu, H. Zhang, et al. Design, synthesis and antifungal activity of novel matrine-hydroxamic acid derivatives containing benzene sulfonamide. RSC Adv, 15 (2025): 16510–16524. [Crossref] [Google Scholar] [PubMed]
- E.L. Kechi, C.B. Ubah, M. Runde, A.E. Owen, O.C. Godfrey, et al. Elucidating the structural basis for the enhanced antifungal activity of amide derivative against Candida albicans: a comprehensive computational investigation. In Silico Pharmacol, 12 (2024): 48. [Crossref] [Google Scholar] [PubMed]
- C.Y. Huang, Y.C. Chen, B.A. Wu-Hsieh, J.M. Fang, Z.F. Chang. The Ca-loop in thymidylate kinase is critical for growth and contributes to pyrimidine drug sensitivity of Candida albicans. J Biol Chem, 294 (2019): 10686–10697. [Crossref] [Google Scholar] [PubMed]
- T. Siswina, M.M. Rustama, D. Sumiarsa, E. Apriyanti, H. Dohi, et al. Antifungal constituents of Piper crocatum and their activities as ergosterol biosynthesis inhibitors discovered via in silico study using ADMET and drug-likeness analysis. Mol., 28 (2023): 7705. [Crossref] [Google Scholar] [PubMed]
- N.C. Silva, J.M. Nery, A.L.T. Dias. Aspartic proteinases of Candida spp.: role in pathogenicity and antifungal resistance. Mycoses, 57 (2014): 1–11. [Crossref] [Google Scholar] [PubMed]
- X. Hu, P. Yang, C. Chai, J. Liu, H. Sun, et al. Structural and mechanistic insights into fungal β-1,3-glucan synthase FKS1. Nature, 616 (2023): 190–198. [Crossref] [Google Scholar] [PubMed]
- J.N. Haro-González, G.A. Castillo-Herrera, M. Martínez-Velázquez, H. Espinosa-Andrews. Clove essential oil (Syzygium aromaticum L. Myrtaceae): Extraction, chemical composition, food applications, and essential bioactivity for human health. Mol., 26 (2021): 6387. [Crossref] [Google Scholar] [PubMed]
- I. Carev, A. GelemanoviÄ, M. Glumac, K. Tutek, M. Dželalija, et al. Centaurea triumfetii essential oil chemical composition, comparative analysis, and antimicrobial activity of selected compounds. Sci Rep, 13 (2023): 7475. [Crossref] [Google Scholar] [PubMed]
- Z. Ma, X. Wang, C. Li. Strategies of drug delivery for deep fungal infection: A review. Pharm Nanotechnol, 8 (2020): 372–390. [Crossref] [Google Scholar] [PubMed]
- D. Kalemba, A. Kunicka. Antibacterial and antifungal properties of essential oils. Curr Med Chem, 10 (2003): 813–829. [Crossref] [Google Scholar] [PubMed]
- D. Klimoszek, M. JeleÅ, M. DoÅowy, B. Morak-MÅodawska. Study of the lipophilicity and ADMET parameters of new anticancer diquinothiazines with pharmacophore substituents. Pharmaceutics, 17 (2024): 725. [Crossref] [Google Scholar] [PubMed]
- I. Khan, W. Rehman, L. Rasheed, F. Rahim, R. Hussain, et al. Discovery of novel and selective Schiff base inhibitors as a key for drug synthesis, molecular docking, and pharmacological evaluation. ACS Omega, 9 (2024): 31148–31158. [Crossref] [Google Scholar] [PubMed]
- Z.Y. Ibrahim, A. Uzairu, G. Shallangwa, S. Abechi. Application of QSAR method in the design of enhanced antimalarial derivatives of azetidine-2-carbonitriles, their molecular docking, drug-likeness, and SwissADME properties. Iran J Pharm Res, 20 (2021): e114536. [Crossref] [Google Scholar] [PubMed]
- A. Daina, O. Michielin, V. Zoete. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep, 7 (2017): 42717. [Crossref] [Google Scholar] [PubMed]
- R.H. Waring. Cytochrome P450: genotype to phenotype. Xenobiotica, 50 (2020): 9–18. [Crossref] [Google Scholar] [PubMed]
- J. Guo, X. Zhu, S. Badawy, A. Ihsan, Z. Liu, et al. Metabolism and mechanism of human cytochrome P450 enzyme 1A2. Curr Drug Metab, 22 (2021): 40–49. [Crossref] [Google Scholar] [PubMed]
- C.R.S. Uppugunduri, Y. Daali, J. Desmeules, P. Dayer, M. Krajinovic, et al. Transcriptional regulation of CYP2C19 and its role in altered enzyme activity. Curr Drug Metab, 13 (2012): 1196–1204. [Crossref] [Google Scholar] [PubMed]
- Y. Guo, Y. Zhang, Y. Wang, X. Chen, D. Si, et al. Role of CYP2C9 and its variants (CYP2C93 and CYP2C913) in the metabolism of lornoxicam in humans. Drug Metab Dispos, 33 (2005): 749–753. [Crossref] [Google Scholar] [PubMed]
- C. Taylor, I. Crosby, V. Yip, P. Maguire, M. Pirmohamed, et al. A review of the important role of CYP2D6 in pharmacogenomics. Genes (Basel), 11 (2020): 1295. [Crossref] [Google Scholar] [PubMed]
- S. Surendran, F. Qassadi, G. Surendran, D. Lilley, M. Heinrich. Myrcene—What are the potential health benefits of this flavouring and aroma agent? Front Nutr, 8 (2021): 699666. [Crossref] [Google Scholar] [PubMed]
- A.I. Krysantieva, J.K. Voronina, D.A. Safin. A novel ambroxol-derived tetrahydroquinazoline with a potency against SARS-CoV-2 proteins. Int J Mol Sci, 24 (2023): 4660. [Crossref] [Google Scholar] [PubMed]
Citation: © 2025 Meriem E, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.