Research Article: Journal of Drug and Alcohol Research (2025) Volume 14, Issue 1
Pathophysiology of Cerebral Cysticercosis Related to Panoptosis, Microglia Polarization and Novel Drugs Implications
Lourdes de Fatima Ibanez Valdes1 and Humberto Foyaca2*2Department of Neurology, Nelson Mandela Academic Hospital, Walter Sisulu University, South Africa
Humberto Foyaca, Department of Neurology, Nelson Mandela Academic Hospital, Walter Sisulu University, South Africa, Email: humbertofoyacasibat@gmail.com
Received: 05-Feb-2025, Manuscript No. JDAR-25-160913; Editor assigned: 10-Feb-2025, Pre QC No. JDAR-25-160913; Reviewed: 24-Feb-2025, QC No. JDAR-25-160913; Revised: 03-Mar-2025, Manuscript No. JDAR-25-160913; Published: 31-Mar-2025, DOI: 10.4303/JDAR/236426
Abstract
Background: Cysticercosis (Ct) is a preventable and eradicable zoonotic parasitic disease secondary to an infection caused by the larva form of pig tapeworm Taenia solium (Ts), in people living/visiting developing countries. However, the number of carriers in developed countries increases gradually due to globalization and uncontrolled migration. When the cysticerci is located in the brain, spinal cord or the optic nerve is called Neurocysticercosis (NCC).
In this study, we look for the role played by Microglia (Mg) and PANoptosis (PANp) in the pathogenesis of NCC. After reviewing this issue, we formulate some hypotheses regarding the role of Mg, trained immune response and PANp in the NCC.
Method: We searched the medical literature comprehensively, looking for published Medical Subject Heading (MeSH) terms like “NCC”, “pathogenesis of NCCs”, “comorbidity in NCC”, OR “apoptosis”, OR “pyroptosis;” OR “necroptosis;” OR “PANoptosis;” OR “PANoptosome;” OR “reprogramming somatic cells;” OR “programmed cell death;” OR “regulated cell death” OR “messenger RNA”.
Results: All selected manuscripts were peer-reviewed and we did not find publications related to Mg/Ap/Pp/Np/PANp/PANop/RSC/NCC.
Comments and concluding remarks: We have hypothesized the role of Mg/PANp on the pathogenesis of cysticercus perilesional oedema and the role of Mg during the colloid/nodular stage of NCC plus new drugs implications.
Keywords
Cysticercosis; Neurocysticercosis; Microglia activation; Apoptosis; Pyroptosis; Necroptosis; PANoptosis; PANoptosome
Introduction
Cysticercosis (Ct) is a preventable neglected zoonosis and eradicable parasitic condition secondary to a cestode invasion of the body by the larva form of the pig tapeworm Taenia solium (Ts), which is most commonly seen in persons living in underdeveloped countries. The most typical clinical features are Epileptic Seizures (ES)/ epilepsy (EP) and headache, among other less frequent medical/surgical conditions [1-5]. ES and Ep are the most common symptoms of Cerebral Cysticercosis (CC). In the past, we performed more than a dozen epidemiological studies in rural areas around Mthatha, confirming that CC, also named Neurocysticercosis (NCC), is the primary aetiology of secondary epilepsy. Most ES and Ep respond well to first-line Antiepileptic Drugs (AED) and Antiseizure Medication (ASM) [6-15]. The most prescribe ASM is benzodiazepine and the commonest used AED are valproic acid and carbamazepine [16-19].
As previously cited, microglia and astrocyte expression are at the centre of NI pathways, either indirectly or directly related to their secretion of proinflammatory elements, including chemokines and cytokines and upregulation of BBB-disrupting proteinases [20-27].
In 2006, we reported the clinical features and other aspects of Spinal Cord NCC (SCNCC) [28]. Recently, we highlighted the role played by pericytes in the pathogenesis of local neuroinflammation due to NCC, the healing process, and the prognosis of SCNCC [29].
Mg contains a small cytoplasm with elongated nuclei of monocyte’s source of mesoderm-derived and they are resident Mp of the CNS involved in the phagocyting/ removing waste metabolite, foreign/damaged organisms, development of brain tissue structure, material or cells [30].
At the previous times of Mg investigations, some investigators considered the presence of two types of Mg known as M1, which release inflammatory factors and toxic substances and inhibit pathogens and M2, which exerts neuroprotective functions by promoting the restoration of impaired immune responses and subsequent nerve repair and cell regeneration. In 1919, Pio del Rio Hortega (Spanish neurologist) named it “satellite” Mg, where the action potential starts [31].
Materials and Methods
To select papers published related to this issue between January 31, 2003 and December 31, 2025, we systematically searched EMBASE, Medline, Cochrane Library, Scopus, PsycINFO, Global Health, CINAHL and Health Management Information Consortium. It was followed by hand-searching relevant journals.
Literature search strategy for this review
We systematically searched investigations published online from January 01, 2000 to January 31, 2023, using PubMed Central and PubMed. First, we screened all papers related to the issues mentioned earlier under the search terms “NCC”, “pathogenesis of NCC”, “comorbidity in NCC”, “apoptosis” or OR “necroptosis,” OR “PANoptosis;” OR “pyroptosis,” OR “PANoptosome;” OR “programmed cell death;” OR “regulated cell death” OR “messenger RNA.” We selected those that were relevant to these issues for review. We also searched for references for all selected papers. Later, we systematically searched the below-mentioned electronic library databases: Health Management Information Consortium, Global Health, Cochrane Library, CINAHL, Web of Science (Clarivate Analytics), EMBASSY, MEDLINE (Ovid) and Scopus (Elsevier) to select the original research studies related to the above search strategy. After a complete peer-review process, the selection criteria were restricted to full-text writing in Spanish, Portuguese and English.
As previously cited, we retrieved all studies using MeSH and included only aspects within the scope of the current work.
Inclusion and exclusion criteria
We also chose randomized clinical trials published in peer-reviewed journals. Studies that assessed other types of parasitic conditions, infections or vascular diseases were excluded because their aetiologies differed from those of T. solium infection.
Quality appraisal
Other areas of study quality were assessed, such as study design, health status, blinding process, selection bias, data collection methods and reasons for dropouts/withdrawals. LdeFIV carried out a methodological quality assessment, which HFS later verified.
Data extraction
A data extraction mechanism was developed to collect research data about patients’ demographic profiles, methods, settings, study design, measurement tools, assessment timing and outcomes. Vital information on the intervention for secondary data analysis studies is extracted from the primary or earlier published articles online.
Methods of analysis
Data syntheses were programmed to comprise the descriptive intervention synthesis of selected manuscripts based on PRISMA’s methodology. Selected data were initially synthesized using textual descriptions to determine the study’s characteristics. Later, they were clustered and presented in tabular form.
Study and cohort selection
We search retrospective and prospective case series, reviews, cohort studies, case-control studies, case reports, cross-sectional studies, controlled clinical trials and metaanalyses. We release data on inclusion criteria, followed by a discussion with our assessor (HFS) to establish consensus on which studies were included in cases of ambiguity.
Data collection process
Each publication’s selected information is processed using Microsoft Excel in a structured coding scheme. The selected data included clinical features, age distribution, population size and the investigations to support the final diagnosis. In situations where we were uncertain regarding the interpretation of the data or how it could be used, we both analyzed the situation until we arrived at a mutual agreement.
Data synthesis
This study used aggregate data following the guidelines of PRISMA.
Quality assessment of selected publications
Initially, all qualified studies were screened for bias using the Jadad scoring system [32], and only those with Jadad scores ≥ 4 were included for further assessment.
Results and Dicsussion
Study selection
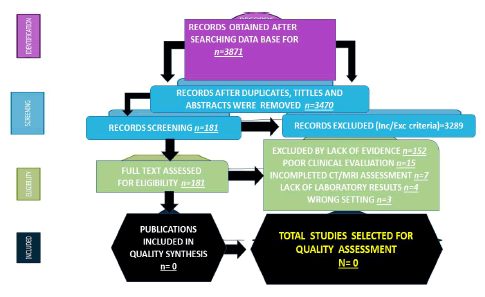
All selected articles were peer-reviewed publications and no one met all inclusion criteria on Mg/Mp/Ap/Pp/Np/ PANop/trained immunology. Below, see a flow chart for the literature searched (Figure 1).
Figure 1: Flow diagram of selected articles (Source: Humberto, 2024).
Study characteristics
Most studies (83.9%) were published in the last three years. The number of people with NCC ranged from 45 342 to 49 671 (median 47 506.5 Interquartile Range (IQR) 12 602–36 610). Most studies were conducted in the Canada/United States of America (42.1%), followed by Asian countries (39.5%), the African continent (12.7) and the European population (5.7%). Most investigations (77.3%) focused on people older than 18 years. The total of publications identified was n=3871; after duplicate removal (n=3470); full text excluded, (n=181); quality synthesis (n=0) and for quality assessment (n=0) were confirmed.
Comments and concluding remarks
Most studies combined case series, reports, literature reviews and immunological analyses. Due to the few studies on children, most clinical features, demographics and immune responses in the diet are missing. Our series identified four stages of cysticercus in the brain, known as: Vesicular: When the parasite is viable with intact membrane, no-host immunological reaction and no Neuroinflammation (NI) around. Colloidal: The dying process of the parasite commonly occurs five years after entry. The intra-cyst fluid becomes turbid (compared with the CSF density, it is darkest). The injured membrane and leaky fluids surround the cyst. In the colloid stage, the neurological features are remarkable due to the direct/ indirect effects of the released glutamate from the parasite’s membrane. Granular nodular: Decreased perilesional oedema and the cyst begins to retract, but the enhancement persists. Calcified: All structural characteristic of the cyst disappears, and the remnant material is calcified [1-29,39]. As we documented before, activation of Mg/Ast and Pc is at the centre of NI pathways either directly or indirectly due to BBB’s upregulation, disrupting proteinase secretion of proinflammatory cytokines and formation of an inhibitory glial scar [19,29]. Future publications will review the aspects of Mg/IS/ES/Ep and NCC-related oxidative stress. However, now we will comment on the relationship between Mg and NCC (NCC) only due to the constriction of several pages for this publication.
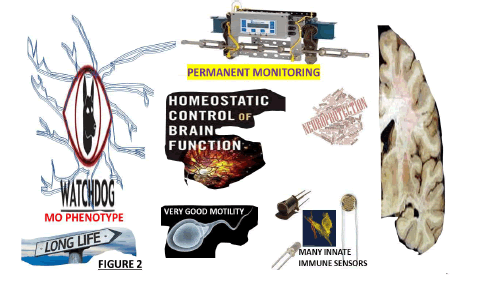
The Mg first proliferate, extending the length of their nuclei, forming aggregates around the necrotic tissues or dying cells and releasing Reactive Oxygen Species (ROS) like P2X7, CB2 and COX-2, which vigorously participate in the pathogenesis of ALS and MS, as we commented before [32]. Here, we are highly aware of some of Mg’s relevant features. As before, resident innate immune cells derive from the nerve tissue’s mesodermal layer. Apart from the homeostatic functions, they prevent overshooting immune responses during health and development, favoured by their prolonged life, remarkable motility and abundant immune sensors. They also are involved in the maturation, development, repair and endogenous immune response by neuroprotective and neurotoxic action of the CNS (Figure 2).
Figure 2:The main features of MO phenotype-resting Mg (Source: Humberto, 2024).
Typically, the Mg has been considered in the permanent resting state (MO phenotype) under normal conditions. Their primary function is to monitor the CNS immunologically by permanently assessing all abnormal activity in the CNS, playing an “immune surveillance and defence” role in the microenvironment of neuron cells and maintaining a relatively quiescent neuron-specific monitoring phenotype. Mg performs a complete assessment of the brain for any injury/damage/infection within very short periods and continuously modulates the necessary interventions to determine the immune status of the brain. Notwithstanding, it has been proposed that the MO implement structural and functional variations under abnormal conditions and polarizes into the typical M1 or M2 alternatively. They rapidly become activated, polarized and accompanied by a series of events such as chemotaxis, phagocytosis, proliferation, migration and proinflammatory cytokine production (interferon-γ, interleukin-1β, tumour necrosis factor-α), proteolytic enzymes, (including heme oxygenase 1), matrix metalloproteinases, a large number of inducible nitric oxide synthase, ROS and Interleukin 12 (IL-12) plus an adaptive immune response, removing foreign pathogens and a sustained inflammatory response aggravating brain injury (Figure 3).
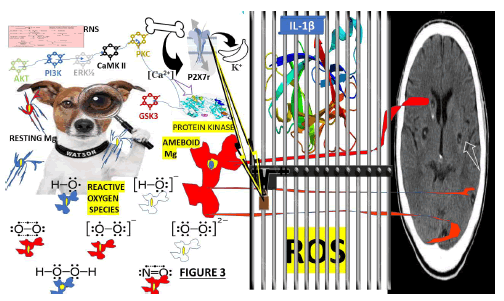
Figure 3:Proposal about the main elements involved in the surveillance of the brain by resting mg during the vesicular stage of NCC during the vesicular stage of NCC including RNS: Reactive Nitrogen Species, are molecules whose main source is nitric oxide and superoxide (O2•−). These are produced through the enzymatic activity of NADPH oxidase and nitric oxide synthase 2; ROS: Reactive Oxygen Species surge from biochemical reactions that occur in Mt, peroxisomes and chloroplasts. It is well known that the mechanism of ATP production in the Mt (oxidative phosphorylation) includes the transport of protons across the inner Mt’s membrane to oxidation-reduction reactions. Included P2X7, CB2, COX2..
During respiration, the Mt convert energy for the cell into a usable form, adenosine triphosphate. The process of ATP production in the Mt, called oxidative phosphorylation, includes the transport of protons across the inner mitochondrial membrane. P2X7: P2X purinoceptor 7 is a protein belonging to the purinoceptors’ family. In cases of cerebral cysticercosis, we hypothesized that after a prudential expression time, it creates a wide pore at the cellular membrane, leading to increased ATP release into the extracellular milieu and PCD. Other investigators have reported that P2X7 receptors are primarily expressed in brain Mg, mainly in the hippocampus, striatum and areas involved in neurodegenerative diseases [82]. We speculated that the P2X7 receptor is highly activated in Mg at the brain-mediated PCD, multinucleated cell production of exosomes, fast and reversible membrane blebbing, phosphatidylserine exposure, the release of microparticles formation of nitrogen species and ROS. In the patients presenting NCC, we hypothesized that the high concentration of intracellular Ca2+ activates some kinases such as PI3K, AKT, GSK3, ERK1/2, CaMKII and PKC, leading to inhibition of Ap or elevated genetic transcription of cell survival. In Figure 3 (as part of this hypothesis), we included the consequences of activating P2X7r, releasing IL-1β/ROS and increasing inhibition of GSK3 if the NLRP3 inflammasome and NF-κB formation are completed. On top of that, we hypothesized that giving P2X7 receptor antagonists to patients with massive NCC instead of PZQ/ ALB/St may improve NI without a risk of developing ES/SE and death. Abbreviations: PKC: Protein Kinase C, CaMKII: Calcium-calmodulin kinase II, PI3K: Phosphorylates and activates phosphoinositide 3-kinase, ERK1/2: Extracellular Signal-Regulated Kinases 1/2, AKT: Protein kinase B, GSK3: Glycogen Synthase Kinase 3.
In intensive brain lesion/damage cases, the Mg sends a signal like “swallow me” followed by an expressed toxic M1/proinflammatory state, typically characterized by amoeboid modifications, infected cells and cytotoxic effects. We hypothesized that M1/M2 Mg perform different actions responding to the NI stages and environmental stimulations.
Comment on M1 phenotype in cerebral cysticercosis
In situations of the sterile NI response (caused by CNS ischemic/reperfusion/trauma) M1 reduce Nicotinamide Adenine Dinucleotide Phosphate (NADPH), secrete Interleukin-6 (IL-6), Interleukin-1α (IL-1α), chemokines and IL-12 plus surface antigen CD40 among other elements with inhibition of phagocytic activities if the production of iNOS, NLRP3 inflammasome or ROS is not enough. Based on the previous information, we have hypothesized that in patients presenting NCC (at the colloid stage), activated M1 Mg secrets, the above-mentioned proinflammatory elements reduce the intensities of the inflammatory process at the pericystic, participate in the process of clearance of waste metabolite area. However, according to the quantity/ quality of the lesion, the M1 phenotype might promote NI and inhibit phagocytosis, causing neuronal injury/ irritation/damage, triggering Ep/ES at the brain cortex and headache due to irritation of the nearby trigeminal receptor in the meninges. Later, this overreaction of M1 Mg leads to increased NI and a delayed nerve-repairing process. The pathogenic process is more complex if associated ischemia/ reperfusion happens. Therefore, we will comment on this aspect in our subsequent publication (Figure 4).
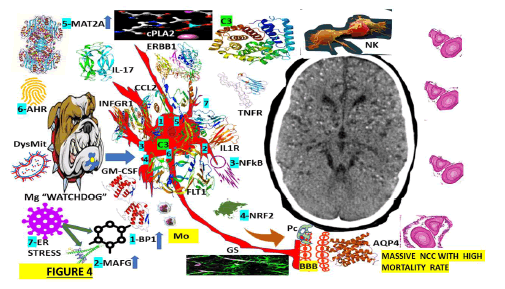
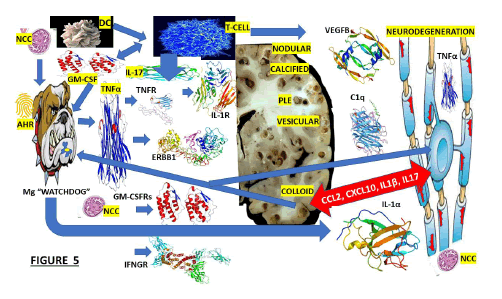
Figure 4: The interaction between the activate Mg and activate Ast during the colloid stage of NCC.
We hypothesized that because of the closed proximity between As and Mg, all supportive cells will be expressed in the brain, even before the immune elements are in the border of the CNS at the meninges and even the choroid plexus. Other authors have documented that As surface receptors, such as IL1R, TNFRs, GM-CSFRs, FLT1, ERBB1, IL-17R, and IFNGRs, are the crucial mediators in this mechanism. The participation of XBP1, activated by endoplasmic reticular stress, is also illustrated in this Figure as another component of this hypothesis, plus the elevation of MAFG, MAT2A and C3 intracellularly. We also speculate that activated As acts as a direct response to activated/M1/Mg, increasing their secretion of proinflammatory molecules as we reported under different circumstances [93] but now including CCL2/GM-CSF, leading to the recruitment of encephalitogenic T-lymphocytes and other proinflammatory monocytes causing NI. OAs illustrated in this Figure, other elements involved in this hypothesis are cPLA2 and NK.
Note: FLT1: Is a member of the VEGF receptor gene family, ERRB1: The epidermal growth factor receptor is a transmembrane protein receptor, IL1R: Interleukin 1 receptor type I, IL-17: Interleukin 17 family is a family of proinflammatory cystine knot cytokines, INFGR1: Interferon Gamma Receptor 1 also called as CD119, cPLA2: cytosolic Phospholipase A2, CCL2: The Chemokine (C-C motif) ligand 2 is also called monocyte chemoattractant protein 1 (MCP1), XBP1: (1) X-box binding protein 1, also called as XBP1, is a transcription factor protein which modulate genes expression, NF-kB: Nuclear factor kappa-light-chain-enhancer protein of activated B cells control cytokine production, and transcription of DNA, C3: Complement component 3, MAT2A: Methionine Adenosyl Transferase is an enzyme that creates S-adenosylmethionine, AHR: The Aryl Hydrocarbon Receptor is a protein transcription factor, BBB: The Blood-Brain Barrier control all circulating solutes, impeding do not crossing into the extracellular fluid to protect neuron cells while allow the entry of the necessary nutrients. CA: Corpora Amylacea participates in the mechanism of clearance of the metabolite waste in the brain; AQP-4: Aquaporin-4 is a water channel protein that participates in the clearance system of the CNS. GS: The Glymphatic System oversees waste clearance in the brain; Mo: Monocytes are a type of white blood cell that can differentiate into monocyte-derived dendritic cells and macrophages. NK: Natural Killer cells (5-20% of all circulating lymphocytes) are cytotoxic lymphocytes of the innate immune system belonging to the innate lymphoid cells family; cpla2: cytosolic phospholipase A2 expression is one of the pathways that activate microglia and astrocytes in the brain, DysMit: Dysfunctional Mitochondria, ER stress: (7) Endoplasmic Reticulum stress is a new apoptosis regulatory pathway. ROS: Reactive Oxygen Species.
Some comments regarding alternative activation (M2 polarization) in NCC
We hypothesized that the released NCC antigen material during the colloid stage led to the activation of Mg cells, induced T-cell activation by myelin autoantigen through IL-17/dendritic cells, activated T-cells to produce proinflammatory cytokine (IL-17/GM-CSF) inducing Mg to promote neurodegeneration, activate transcription factor NF-kB and expression of transcription factor hydrocarbon receptor (AHR), which is downregulated and Mg secret/ upregulate IL-1α, TNF-α, C1q, IL-1β and VEGFB.
Some authors argue that Mg polarization (based on M1/ M2 phenotypes) is still not well proven, while other investigators postulate the contrary [31].
Based on Del Rio Hortega’s publication, we hypothesized that “Satellite” Mg exists in the thalamus, hippocampus and cerebral cortex and we assumed those cells are present in the colloid stage of NCC when the parasite is dying due to natural causes or because of the effect of anti-parasitic medicine. Due to its preferential location, it may influence the mechanism of electrical neurotransmission and it has been proven to interact with the proximal dendrites of neuron cells in the cerebral cortex of monkeys [33,34]. Therefore, we hypothesized that “satellite” Mg might play a vital role in the pathogenesis of ES/Ep of patients with NCC.
KSPG Mg is in the brainstem/hippocampus. Therefore, we do not expect it to play any role in the pathogenesis of ES/ Ep/IS in NCC. Another type of Mg is Hoxb8, which shows different molecular characteristics and spatial distribution compared to typical Mg. Because it is located in the brain cortex, functional loss may affect the corticostriatal tract and lead to abnormal social behaviour [35].
We believe that the status of Mg (M1/M2) expression could not be established by their typical markers (Arg1, CD11b, CD206, TGF-β, TNF-α and iNOS). We expect that further studies on Mg will support our hypotheses and bring more clarifications on the functions/activities of Mg-based on results reported from transcriptomics techniques RNA such as proteomics, neuroimaging techniques, transcriptomics, computational biology, new animal models, epigenetics, mass spectrometry cell analysis, single-cell sequencing techniques as multi-omics methods, immunohistochemical methods (with high-resolution laser ablation), patientderived Mg plus a combination of other novel procedures that will serve to bring more clarification to the unknown issues related to Mg phenotypes/polarization and at the end obtain more in-depth understanding of Mg as the most dynamic supporting cell of the healthy/ mature CNS.
Some comments on the Mg states and nomenclature for NCC
Most authors have been working on Mg terminology for the past ten years, mainly “M1 versus M2” or “resting Mg versus activated Mg”, separating Mg into deficient or good Mg. Notwithstanding, it is well accepted that Mg has many other states and functions in CNS disorders, such as ageing, development and neuroplasticity, compared to those we considered before. Some authors described several Mg’ states and models such as Mg Neurodegenerative phenotype (MgNd), Interferon-Responsive Mg (IRMg), Disease- Associated Mg (DAMs) mainly in connection with AD, activated responsible Mg, human AD Mg, PD Mg signature, Mg inflamed in MS, ALS-associated signature, gliomaassociated Mg, lipid-droplet-accumulating Mg, white matterassociated Mg and axon tract-associated Mg [37]. Based on these reports, we hypothesized that there are other states/ models of Mg in several locations of NCC that might be called Mg NCC instead of M1/M2/active/resting Mg, that needs resident CD4+ T cell for acceptable transition/maturation responding to the influence of NK cells, T/B lymphocytes and other immune cells changing their molecular profile, motility, morphology, ultrastructure and function according to the modifying factors before-mentioned. Forthcoming studies using the P2RY12 Mg marker might determine the differences between Mg and Mp in NCC and their role in NI/neuroplasticity. Other well-designed future investigations could clarify the exact role of Mg in the process of NI in the brain during the colloid states of NCC.
Recently, Dermitzakis and colleagues confirmed the previous postulates above-cited and highlighted the role of Mg as a tissue-resident Mp primary innate immune cell in the CNS and its biological role in maintaining homeostasis by removing viruses, bacteria and other infection material. Mg is the only tissue-resident Mp which differs from their Mp-hematogenous source due to their immune-privileged environment owing to the construction of the BBB [38] and supported by the glymphatic system and AQP4/CA/As (IL- 33) [39]. We also considered that Mg in the brain needs TGF-β to keep its capacity of surveillant on surrounding tissues and an impact on its development from E14.5. As has been reported by other authors under different pathological conditions [40].
Recently, we commented on the role of micro-ribonucleic acid (miRNA) in viral infections [41]; now we hypothesized that glutamate released by cysticercus during the colloid stage of NCC dysregulated functional miRNA (produced by neurons/GC), targeting several proteins leading to deregulate supporting/neuron cell signalling, PCD gene expression, innate neuroimmune response and modulate the messenger RNA. We considered miRNA a crucial diagnostic element and the therapeutic molecule for curative therapy for many diseases [41]. In cases of NCC, the depletion of miR-124 triggers Mg activation. Unfortunately, we do not get enough confidence information to elaborate a sustainable hypothesis about it.
At the same time, Mg has the privilege of staying near the neuron community at the brain parenchymal as the only immune cells [42], addressing the surveillance of all neuron cell activities and like a dwarf orchestra’s Director, guiding the compass of a giant cell group’s participation in almost all CNS disorders, as we believe.
Brief comments on Apoptosis (Ap), Pyroptosis (Pp), Necroptosis (Np) and PANoptosis in NCC
It is classically accepted that Np is a programmed form of inflammatory cell necrosis/death differentiated from Ap due to a lack of participation in caspase activation from released Mt cytochrome C/leakage of cell debris into the extracellular space.
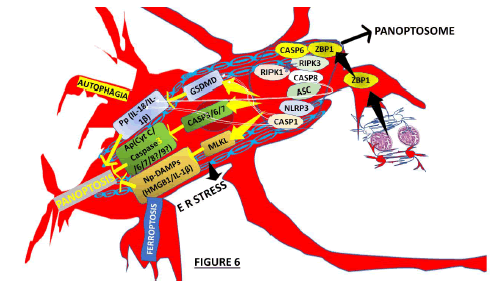
Based on other publications [81,82], we hypothesized that the brain Mg could identify the signals produced by NCC/ colloid antigens over the surrounding cells and respond fast, producing proinflammatory elements and activating PCD mechanisms Ap including, Pp, Np, autophagy ferroptosis and. According to the number/locations/stage of NCC in the brain parenchyma plus the quality of the host’s immune response (proinflammatory cytokines/chemokines), then many types of PCD might be seen in isolated or combined presentation of PANp and Np attenuating the severity of the infection, as we represented in Figure 6.
We hypothesized that in NCC, both Np and Pp are lytic forms of PCD modulated by mixed lineage kinase domainlike expression, which mediates Pp and releases intracellular cytokine and Damage-Associated Molecular Patterns (DAMPs). Simultaneously, Np happens after receptorinteracting serine/threonine-protein kinase 3-dependent phosphorylation, producing disruption of membrane integrity of MLKL. PANoptosis is the only PCD regulated by the PANoptosome (Figure 6) [82].
In 2019, Malireddi et al. proposed the term PANoptosis and described a novel PCD mechanism by the interaction of Pp, Ap and Np. The results cannot be explained by any of them separately [84].
Figure 5 shows the ER stress as a novel Ap regulatory pathway in cases where ER stress is present due to NCC and where the system will respond with an unfolding protein response expressed by protein kinase R-like ER kinase -PERK, Activating Transcription Factor 6 (ATF6) and IRE1 (Inositol-Requiring Enzyme 1) which are ER stress integrated proteins [84]. As illustrated in Figure 5, RIP3 phosphorylates MLKL protein soon after, which activates NLRP3 inflammasome and promotes translocation to the cell membrane, producing MLKL oligomers, leading to programmed/regulated cell death in patients presenting NCC. Now, each cell death modality is strongly linked and one is regulated by another and vice versa, as reported [86]. Because the therapy with an Ap inhibitor might suppress Pp and activate Np, it has been considered that a Pp inhibitor can then suppress Pp and activate Np [87].
Figure 5: Graphical representation of the PANoptosis and it PCD/RCD.
Based on the results released by Shi et al. [84], we speculated on the relationship between the molecular switch of cell death, known as caspase-8 (Cp-8) and Ap, Pp (Cp-3, 8) and Np as the regulator. Nevertheless, due to a lack of sustainable evidence, we keep this hypothesis out of inflammatory caspases (Cp-1, 4, 5 and 11). However, we keep another relevant molecular regulator of Np, Pp and Ap in our hypothesis based on its capacity to interact with Pp and Ap [88]; activated RIP3 also releases IL-1β via the NLRP3-caspase-1 axis, which supports that RIP3 and play a vital role in modulating the equilibrium between Pp, Ap and Np, as we illustrated in Figure 6, where the process of PANp is summarized.
Figure 6: Graphical representation of the PANoptosis and it PCD/RCD.
We will comment on Inflammasome (Inf) and PANopsome (PANop), respectively and their role in the pathophysiology of NCC. However, before that, we would like to highlight the Z-DNA-Binding Protein (a nucleic acid innate immune sensor) required to activate NLRP3 and PANoptosis, whose primary activity is to regulate the immune response of the affected cases. Its principal function is to trigger Np, Ap and Pp through RIP3, NLR thermal protein domain associated protein three and Cp-8 expression, being it a crucial component of PANop, which is composed of adapters (apoptosis-associated speck-like protein containing a CARD and FADD), receptors (ZBP1, INfs and its Zα2 domain), and catalytic effectors (RIP1, caspase-1, RIP3 and caspase-8) [84]. On the other hand, it has been well documented that ZBP1 is upregulated in patients infected by COVID-19. We also speculate about the role played by RIP1 regulating PANop for cell death and NI response, releasing antigens from the colloid stage of NCC. Yersinia must modulate the components of the PANop complex, such as FADD, caspase 8, NLRP3, RIP1, ASC and RIP3, to control the PANop pathways, as has been reported under different circumstances [92]. We hypothesized that a therapeutic procedure able to inactivate caspase-8/RIP3 or even FADD/RIP3 might protect the cellular population’s host defence mechanism from cell death and collateral damage. Based on other reports (made under different scenarios), we also considered that almost all recognized Inf sensors (NLRP3, NLRP1, Pyrin, and NLRC4) are involved in PANop [84], as we represented graphically in Figure 6. In our hypotheses, we considered that NCC antigens activate Mg/Mp, leading to upregulated expression of caspase-3, GSDMD, caspase-1, RIP3, MLKL, IL-1β, IL-18, p-MIKL and NLRP3 in Mg/Mp inducing PCD according to the stage of NCC. All cystic lesions are not at the same stage because all cysticerci do not invade the brain simultaneously. In cases with NCC, the inhibition of TLR9 might suppress the MAPK pathway/PANop and improve prognosis, as reported in other pathological processes [87].
One relevant aspect of NCC is the parasite’s ability to evade/suppress the host innate immunity function of Mg by releasing abundant Excretory/Secretory Proteins (ESPs). ESPs are a heterogeneous group of glycoproteins, proteins and other necessary molecules that allow the vesicular stage of NCC to survive and remain healthy in the host’s brain under closed surveillance of Mg.
Based on these previous arguments, we hypothesized that all types of PCD can be present in multifocal colloid NCC, where many participating factors emerge from the interrelationship between Pp, Ap and Np. Therefore, we believe that the predominant PCD modality in NCC is accompanied by increased production of microbicidal molecules (ROS and NO) and gene expression, as we commented in previous articles [12,16,17,39,81, 89-93].
According to our proposal, the NCC colloid stage is closely associated with PANop as a predominant Regulatory Cell Death Programme (RCDP). However, another RCDP characterized by iron-dependent lipid peroxidation called Fp might be involved in the pathophysiology of NCC. Fp is associated with COVID-19, tumour progression, malaria, tuberculosis and cryptococcal meningitis [94]. We reported a patient presenting a comorbidity of NCC/COVID-19, a high level of ferritin (a crucial protein for storing iron) avoiding cell injury caused by the Fenton reaction, presenting a brainstem dysfunction [16].
Some comments on somatic NC reprogramming in cerebral cysticercosis.
Some investigators have proposed that Somatic Cell Reprogramming (SCR) is an alternative way to get new cells based on novel techniques for reprogramming differentiated and mature cells into NC [95].
These neural stem cells are scanty; therefore, they cannot replace the large numbers of neurons lost during massive NCC as they might when there are few brain cysticercosis lesions. The exact number of cysts has not been identified yet [96].
These studies documented that the nuclei of adult cells could be reprogrammed and that they exhibited cellular characteristics like those of fertilized eggs. Oher’s authors documented that the nuclei of adult cells may be reprogrammed into embryonic stem cells and that these stem cells may be differentiated into different types of cells [12]. Several kinds of cells from other tissues and embryonic cortices participate in reprogramming somatic cells, mainly ectodermal cell types such as fibroblast, OLG, Ast, keratinocytes, NC and Pc [97]. These authors concluded that it is happening because embryonic cells and progenitor cells may differentiate into cells with the same genealogical sources; therefore, neuronal ectoderm can produce Pc, As and fibroblasts, but As are the best candidates for neuroregenerative reprogramming on top of Mg [97] that is why we hypothesized that from nodular to the calcified stage most of new neurons cells replacing those lost during the PCD mechanism arise from Ast/RSC.
We hypothesized that Mg might be potentiated in responding to nonspecific attacks against new cysts invading CNS tissues from new ingestion of eggs/proglottids of T. solium via an innate type of immunological memory, also named trained immunity, also considered as a new weapon in the fight against the infectious process, as has been documented by other investigators [98]. We speculated that Mg has an innate immunological memory, which is expressed soon after exposure to T. solium’s antigens, as has been postulated by other authors (under different circumstances) [99]. Other investigators have established that Mg may change the epigenetic landscape chromatin structure across this process, diminishing DNA methylation, activating proinflammatory genes and increasing histone methylation and acetylation marks [100].
As we cited recently, PANp has emerged as a relevant contributor to the pathogenesis of some known neurological conditions such as AD, PD and ALS, combining the effect of apoptosis, autophagy, pyroptosis, necroptosis, ferroptosis and cuproptosis; some of them are modulated by multifaceted PANoptosome complexes (a cytoplasmic protein complex), Plus Oxidative Stress (ROS-triggering PANp) and dysfunctional mRNA within the neuron cells at the pericystic region responding to excreted glutamate during the colloid stage of NCC [101,102]. Therefore, we hypothesized about the role played by glutamateinduced programmed cell death in the hippocampal neuronal population leading to Epileptic Seizures (ES) and Secondary Epilepsy (SEp). Based on the presence of many biomarker genes in patients presenting NCC, such as RIPK1, CASP1, CASP6, CASP6, CASP8, IL18, MLKL, IRF1, GSDMD, ZBP1, PYCARD and their predictive ability and great diagnostic accuracy for epilepsy reported by other authors [103], we strongly support our previous hypothesis. The pathogenesis of the colloid stage of NCC involves NI, blood-brain barrier disruption and PCD/RCD, with NI being the core process. Nogo-A (a neurite growthinhibitory protein in the central nervous system) [104].
As we documented before [104] and have been supported by other authors [105], neuroinflammation is a multifaceted and complex process, including dynamic interactions among molecular components and various cellular processes. The well-organized interplay supports system resilience and environmental adaptability, as seen in cases of NCC at the vesicular stage in the brain, which is severely disrupted during NI. We also described the most relevant components of the cited neuroimmune process, such as Mg, As, neurons, immune cells, chemokines, cytokines, neurotransmitters, neurotrophic factors and extracellular matrix components. We hypothesized the mechanism of neuroimmune interactions, mediated through these signalling pathways and cellular actors, creating a complex network that modulates CNS functionality to respond to the colloid stage of NCC and subsequent NI [104].
Inhibition of Mg/Mt fragmentation by Mt division inhibitor 1 (Mdiviâ?1) alleviates OS, pyroptosis and NI through p62 enhancement and NFâ?κB, Keap1/Nrf2/HOâ?1 pathways, respectively [106].
Microglia play a vital role in the development of NI and are closely related to cognitive dysfunction in infections.
Recently, we reported that Mp polarization is modulated by Mt metabolism during the colloid stage of NCC, which is strongly related to Mt fission. This stage exhibits remarkable Mt fragmentation during the dying process of cysticercus [104].
Finally, we hypothesized that advancing gene therapy, novel drug candidates like Mdiviâ?1 and drug interventions targeting Nogo-A protein (Rtn4A) pathways might alleviate hippocampal Mg/Mt excessive fission and suppress its polarization, expression, NI, OS and RCD (pyroptosis) during the colloid stage of NCC via increasing p62 and autophagosome formation plus the Keap1/Nrf2/HOâ?1 and NFâ?κB and pathways as has been supported by another investigator under similar pathological conditions [106].
We hypothesized that in the colloid stage of NCC, there is damage to the blood-brain barrier, accompanied by NI and PCD/RCD, as can be seen in sepsis-associated encephalopathy without direct brain infection, reflecting injury to multiple key brain areas and neural networks as reported Lui et al. in cases inducing ROS and M1 polarization like NCC [104]. We documented before that M1 polarization of Mg activates NF-κB through ROS production, releasing inflammatory elements like interleukin IL-6, IL-1β and TNF-α, leading to chronic NI. On top of that, high levels of ROS lead to PCD/RCD, including Mg, damaging the function and integrity of neural networks. Nogo-a may influence neuroprotection, and NI controls the MG expression, which may inhibit the excessive expression of Mg, diminish the release of inflammatory mediators and thus alleviate the Nc/glial damage extension.
Recently we established that the Mt-associated Endoplasmic Reticulum (ER) membrane is a crucial subcellular structure formed by the juxtaposition of ER subdomains with the outer Mt membrane and among its function are Calcium Ion (Ca2+) signalling and transport, lipid transfer, metabolism, mitochondrial dynamics, PCD/RCD (DNA fragmentation and phosphatidylserine translocation), maintain protein homeostasis and regulation of PANoptosis formation and expression [19]. On the other hand, changes in ER integrity may lead to adverse effects [104] and elevation of calcium ions concentration can lead to increased Mt ROS, which transfers from the ER to the Mt, causing Mt damage and releasing Mt components into the cytoplasm as damage-associated molecular patterns, rapidly activating the NLRP3 inflammasome residing in the ER membrane [19,29]. Several elements, including potassium ion efflux, lysosomal disruption, Mt dysfunction and degradation across the Golgi apparatus in most tissues, express NLRP3 inflammasome. However, it is mainly activated in Mg, which increases Mt ROS from Mt damage. PERK (an important component of ER) also augment the DNA-binding expression of NF-κB with subsequent TNFα activation and modulation of Mt-related OS and potassium efflux-induction of mtDNA release activates the NLRP3 PANoptosis. The MAPK expression is also related to high glucose-induced Mt inflammation [19,29,104].
Conclusion
To the best of our knowledge, this review is the first to propose an interaction between excreted glutamate-induced PCD/RCD during the colloid stage of NCC, leading to PANp/hippocampal neuronal cell death/ES/SEp and the role played by the Nogo-A protein in Mg expression. However, other studies must be performed to confirm/ reject/modify our hypotheses.
Consent for Publication
We did not request written informed consent for publication because this study is not suitable for that requirement.
Ethical Approval
The WSU/NMAH Ethical Committee did not ask for ethical approval for this study.
Competing Interest
The author declares that she performed this study without any commercial, financial or otherwise relationships able to construe a potential conflict of interest.
Funding
The author declares that she did not receive financial aid or collaboration that could have influenced the results reported in this paper.
Author Contributions
Conceptualization-LFIB; writing-review and editing LFIV, artwork, LFIV; supervision and project administration, LFIV and HFS. The author has read and agreed to the published this version of the manuscript.
Declaration of Anonymity
The author certified that she did not mention names, initials and other identity issues of any patient. Therefore, a complete anonymity is guaranteed.
Availability of Data and Material
All data supporting this study are available on reasonable request from the corresponding author.
Acknowledgement
Special thanks to Prof Humberto Foyaca Sibat for his participation in the management of our patients and supervision of this study.
References
- H. Foyaca-Sibat, L. Ibanez-Valdes, Pseudo seizures and epilepsy in neurocysticercosis, Electron J Biomed, 2(2003):20-29.
- H. Foyaca-Sibat, L. Ibanez-Valdes, Vascular dementia type binswanger’s disease in patients with active neurocysticercosis, Rev Electron Biomed, 1(2003):32-42.
- H. Foyaca-Sibat, L. Ibanez-Valdes, Insular neurocysticercosis: Our finding and review of the medical literature, J Neurol, 2(2006):31-35.
- L.D. Foyaca-Sibat H Cowan, H. Carabin, G. Serrano-Ocana, R.C. Krecek, A. Willingham, Accuracy of serological exam for the diagnosis of neurocysticercosis in outpatients with epilepsy, Eastern Cape Province, South Africa, PLOS Neglected Trop Dis, 3(2009):1-7.
- H. Foyaca-Sibat, L. Ibanez-Valdes, J. More-Rodriguez, Parasitic zoonoses of the brain: Another challenger?, J Neurol, 12(2009).
- H. Foyaca-Sibat, L. Ibanez-Valdes, Treatment of epilepsy secondary to neurocysticercosis. InNovel Treatment of Epilepsy, (2011).
- H. Foyaca-Sibat, L. Ibanez-Valdes, Clinical features of epilepsy secondary to neurocysticercosis at the insular lobe, InNovel Aspects on Epilepsy, (2011).
- H. Foyaca-Sibat, Epilepsy secondary to parasitic zoonoses of the brain, InNovel Aspects on Epilepsy, (2011).
- H. Foyaca-Sibat, M. Salazar-Campos, L. Ibanez-Valdes, Cysticercosis of the extraocular muscles. Our experience and review of the medical literature, Internet J Neurol, 14(2012).
- H. Foyaca-Sibat, L. Ibanez-Valdes, Novel aspects on cysticercosis and neurocysticercosis, BoD–Books on Demand, (2013).
- H. Foyaca-Sibat, L. Ibanez-Valdes, What is a low frequency of the disseminated cysticercosis suggests that neurocysticercosis is going to disappear?, InNovel Aspects on Cysticercosis and Neurocysticercosis, (2013).
- H. Foyaca-Sibat, L. Ibanez-Valdes, Uncommon clinical manifestations of cysticercosis, In Novel Aspects on Cysticercosis and Neurocysticercosis, (2013).
- H. Foyaca-Sibat, L. Ibanez-Valdes, Psychogenic nonepileptic seizures in patients living, Seizures, (2018):97.
- H. Foyaca-Sibat, L. Ibanez-Valdes, Subarachnoid cysticercosis and ischaemic stroke in epileptic patients, Seizures, (2018):154-173.
- E.V. Noormahomed, F.S.H. Noormahomed, E. Virgínia, N. Nhancupe, J. Mufume, Neurocysticercosis in epileptic children: An overlooked condition in mozambique, challenges in diagnosis, management and research priorities, EC Microbiol, (2021):49.
- H. Foyaca Sibat, Racemose neurocysticercosis long COVID and brainstem dysfunction: A case report and systematic review, Clin Schizophr Relat Psychoses, (2021).
- H. Foyaca Sibat, Neurocysticercosis, epilepsy, COVID-19 and a novel hypothesis: Cases series and systematic review, Clin Schizophr Relat Psychoses, (2021).
- H.F. Sibat, Comorbidity of neurocysticercosis, HIV, cerebellar atrophy and SARS-CoV-2: Case Report and systematic review, Clin Schizophr Relat Psychoses, 15(2021).
- H. Foyaca-Sibat, L. Ibanez-Valdes, Novel hypotheses on the role of oligodendrocytes in neurocysticercosis. Comprenhensive review, Clin Schizophr Relat Psychoses, 17(2023).
- H. Foyaca-Sibat, Comorbidity of neurocysticercosis, HIV, cerebellar atrophy and SARS-CoV-2: Case report and systematic review, Clin Schizophr Relat Psychoses, 15(2021).
- H. Foyaca-Sibat, A.H. Del Rio-Romero, L. Ibanez-Valdes, E. Vega-Novoa, Neuroepidemiological survey for epilepsy and knowledge about neurocysticercosis at Ngqwala location, South Africa, Internet J Neurol, 3(2005).
- H. Foyaca-Sibat, D.R. Romero, L. Ibanez-Valdes, Prevalence of epilepsy and general knowledge about neurocysticercosis at Ngangelizwe location, South Africa, The Internet J Neurol, 4(2005).
- A. Del Rio, H. Foyaca-Sibat, L. IbaNez-ValdEs, Epidemiological survey about socio-economic characteristic of Mpindweni location, South Africa, Internet J Neurol, 4(2005).
- H. Foyaca-Sibat, L. Ibanez-Valdes, Refractory epilepsy in neurocysticercosis, The Internet J Neurol, 5(2006):34-41.
- H. Foyaca-Sibat, L. Ibanez-Valdes, Insular neurocysticercosis: Our finding and review of the medical literature, Internet J Neurol, 2(2006):31-35.
- H. Foyaca-Sibat, L. Ibanez-Valdes, Combined central and peripheral demyelinating spectrum disorder: A case report and systematic review, Clin Schizophr Relat Psychoses, 17(2023).
- H. Foyaca-Sibat, L. Ibanez-Valdes, Co-morbidity of spinal cord neurocysticercosis and tuberculosis in a HIV-positive patient, Internet J Neurol, 7(2006):5-10.
- H. Foyaca-Sibat, L. Ibanez-Valdes, The role of pericytes in neurocysticercosis comprehensive research and novel hypotheses, Clin Schizophr Relat Psychoses, 3(2023).
- P.E. Ludwig, J.M. Das, Histology, glial cells, InStatPearls, StatPearls Publishing, 2023.
- J. Wang, H. Wenbin, Z. Junlong, A richer and more diverse future for microglia phenotypes, Heliyon, 9(2023).
- A. Jadad, Assessing the quality of reports of randomized clinical trials: Is blinding necessary?, Control Clin Trials, 17(1996):1-12.
- M. Hanani, A. Verkhratsky, Satellite glial cells and astrocytes, a comparative review, Neurochem Res, 46(2021):2525–2537.
- J.H. Lee, W. Kim, The role of satellite glial cells, astrocytes and microglia in oxaliplatin-induced neuropathic pain, Biomedicines, 8(2020):324.
- D. Shrutokirti, D.V. Deren, E. Peden, M. Hockin, A. Boulet, et al. Two distinct ontogenies confer heterogeneity to mouse brain microglia, Development, 145(2018):dev152306.
- T. Kohno, K. Ryoji Shirasaka, K. Yoshihara, S. Mikuriya, K. Tanaka, et al. A spinal microglia population involved in remitting and relapsing neuropathic pain, Science, 376(2022):86-90.
- R.C. Paolicelli, Microglia states and nomenclature: A field at its crossroads, Neuron, 110(2022):3458-3483.
- I. Dermitzakis, M.E. Manthou, S. Meditskou, M.E. Tremblay, S. Petratos, Origin and emergence of microglia in the CNS-an interesting (Hi) story of an eccentric cell, Curr Issues Mol Biol, 45(2023):2609-2628.
- H. Foyaca-Sibat, L. Ibanez-Valdes, Combined central and peripheral demyelinating spectrum disorder: A case report and systematic review, Clin Schizophr Relat Psychoses, 17(2023).
- M. Prinz, T. Masuda, A. Michael Wheeler, J.Q. Francisco Prinz, Microglia and central nervous system–associated macrophages-from origin to disease modulation, Annu Rev Immunol, 39(2021):251-277.
- H. Foyaca-Sibat, L. Ibanez-Valdes, S. Joseph, Comorbidity of human herpes virus, human immunodeficiency virus and polyneuropathy, Clin Schizophr Relat Psychoses, 17(2023).
- P. Marco, J. Priller, The role of peripheral immune cells in the CNS in steady state and disease, Nat Neurosci, 20(2017):136-144.
- L. Shuailong, I. Wernersbach, S.H. Gregory, M. Schafer, Microglia subtypes show substrate-and time-dependent phagocytosis preferences and phenotype plasticity, Front Immunol, 13(2022):945485.
- T. Wan, Y. Huang, X. Gao, W.U. Wanpeng, W. Guo, Microglia polarization: A novel target of exosome for stroke treatment, Front Cell Dev Biol, 10(2022).
- R.M. Ransohoff, A polarizing question: Do M1 and M2 microglia exist?, Nat Neurosci, 19(2016):987-991.
- G. Shenrui, H. Wang, Y. Yafu, Microglia polarization from M1 to M2 in neurodegenerative diseases, Front Aging Neurosci, 14(2022):815347.
- A. Michelucci, T. Heurtaux, L. Grandbarbe, E. Morga, P. Heuschling, Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: Effects of oligomeric and fibrillar amyloid-beta, J Neuroimmunol, 210(2009):3–12.
- C.D. Mills, K. Kincaid, J.M. Alt, M.J. Heilman, A.M. Hill, M-1/M-2 macrophages and the Th1/Th2 paradigm, J Immunol, 164:6166-6173.
- O.M.P. Butovsky, C.S. Jedrychowski, R. Cialic, J.L. Amanda, G. Gabriely, et al. Identification of a unique TGF-β–dependent molecular and functional signature in microglia, Nat Neurosci, 17(2014):131-143.
- F.O. Martinez, S. Gordon, The M1 and M2 paradigm of macrophage activation: Time for reassessment, F1000Prime Rep, 13(2014):6-13.
- R.M. Ransohoff, A polarizing question: Do M1 and M2 microglia exist?, Nat Neurosci, 19(2016):987–991.
- W.J. Streit, M.B. Graeber, G.W. Kreutzberg, Functional plasticity of microglia: A review, Glia 1, 5(1988):301–307.
- L. Acarin, J.M. Vela, B. Gonzalez, B. Castellano, Demonstration of poly-N-acetyl lactosamine residues in ameboid and ramified microglial cells in rat brain by tomato lectin binding, J Histochem Cytochem, 42(1994):1033-1041.
- T. Kitamura, T. Miyake, S. Fujita, Genesis of resting microglia in the gray matter of mouse hippocampus, J Comp Neurol, 226(1984):421-433.
- M.E. Tremblay, C. Lecours, L. Samson, V. Sanchez-Zafra, A. Sierra, From the Cajal alumni Achucarro and Rio-Hortega to the rediscovery of never-resting microglia, Front Neuroanat, 9(2015):45.
- M.E. Tremblay, The role of microglia at synapses in the healthy CNS: Novel insights from recent imaging studies, Neuron Glia Biol, 7(2011):67-76.
- U.K. Hanisch, H. Kettenmann, Microglia: Active sensor and versatile effector cells in the normal and pathologic brain, Nat Neurosci, 10(2007):1387–1394.
- M.E. Tremblay, C. Madore, M. Bordeleau, L. Tian, A. Verkhratsky, Neuropathobiology of COVID-19: The role for Glia, Front Cell Neurosci, 14(2020):592214.
- A. Sierra, M.E. Tremblay, H. Wake, Never-resting microglia: Physiological roles in the healthy brain and pathological implications, Front Cell Neurosci, 8(2014):240.
- K. Borst, A.A Dumas, Prinz M, Microglia: Immune and non-immune functions, Immunity, 54(2021):2194–2208.
- C. Bottcher, S. Schlickeiser, M.A.M. Sneeboer, D. Kunkel, A. Knop, Human microglia regional heterogeneity and phenotypes determined by multiplexed single-cell mass cytometry, Nat Neurosci, 22(2019):78–90.
- H. Kalkman, F. Dominik, Microglia M2A polarization as potential link between food allergy and autism spectrum disorders, Pharmaceuticals, 10(2017):95.
- C. Yunna, H. Mengru, W. Lei, C. Weidong, Macrophage M1/M2 polarization, Eur J Pharmacol, (2020):877.
- S. Jander, G. Stoll, Strain-specific expression of microglial keratan sulfate proteoglycans in the normal rat central nervous system: Inverse correlation with constitutive expression of major histocompatibility complex class II antigens, Glia, 18(1996):255-260.
- D. Trankner, A. Boulet, E. Peden, R. Focht, D. van Deren, et al. A microglia sublineage protects from sex-linked anxiety symptoms and obsessive compulsion, Cell Rep, 29(2019):791–799.
- K. Young, H. Morrison, Quantifying microglia morphology from photomicrographs of immunohistochemistry prepared tissue using Image, J Vis Exp, (2018).
- B.I. Arioz, B. Tastan, E. Tarakcioglu, K.U. Tufekci, M. Olcum, et al. Melatonin attenuates LPS-induced acute depressive-like behaviors and microglial NLRP3 inflammasome activation through the SIRT1/nrf2 pathway, Front Immunol, 10(2019):1511.
- J. Xu, Z. Chen, F. Yu, H. Liu, C. Ma, et al. IL-4/STAT6 signaling facilitates innate hematoma resolution and neurological recovery after hemorrhagic stroke in mice, Proc Natl Acad Sci, 117(2020):32679–32690.
- L. Subedi, J.H. Lee, S. Yumnam, E. Ji, S.Y. Kim, Anti-inflammatory effect of sulforaphane on LPS-activated microglia potentially through JNK/AP-1/NF-κB inhibition and Nrf2/HO-1 activation, Cells, 8(2019):194.
- V. Stratoulias, T.I. Heino, MANF silencing immunity induction or autophagy trigger an unusual cell type in metamorphosing Drosophila brain, Cell Mol Life Sci, 72(2015):1989–2004.
- Kisucka, K. Bimbova, M. Bacova, J. Galik, N. Lukacova, Activation of neuroprotective microglia and astrocytes at the lesion site and in the adjacent segments is crucial for spontaneous locomotor recovery after spinal cord injury, Cells, 10(2021):1943.
- D. Zhou, L. Ji, Y. Chen, TSPO modulates IL-4-induced microglia/macrophage M2 polarization via PPAR-γ pathway, J Mol Neurosci, 70(2020):542-549.
- R. Hardeland, Melatonin and microglia, Int J Mol Sci, 22(202):8296.
[Crossref] [Google Scholar] [PubMed]
- C.F. Tsai, G.W. Chen, Y.C. Chen, C.K. Shen, D.Y. Lu, et al. Regulatory effects of quercetin on M1/M2 macrophage polarization and oxidative/antioxidative balance, Nutrients, 14(2021):67.
- J.P. Louboutin, D.S. Strayer, Relationship between the chemokine receptor CCR5 and microglia in neurological disorders: Consequences of targeting CCR5 on neuroinflammation, neuronal death and regeneration in a model of epilepsy, CNS Neurol Disord Drug Targets, 12(2013):815-829.
- K. Borst, A.A. Dumas, M. Prinz, Microglia: Immune and non-immune functions, Immunity, 54(2021):2194–2208.
- M. Hanani, A. Verkhratsky, Satellite glial cells and astrocytes, a comparative review, Neurochem Res, 46(2021):2525–2537.
- J.H. Lee, W. Kim, The role of satellite glial cells, astrocytes and microglia in oxaliplatin-induced neuropathic pain, Biomedicines, 8(2020):324.
- F. Sibat, Humberto haemorrhage and blindness in times of the coronavirus pandemic and dysbiosis: Case report and literature review, Clin Schizophr Relat Psychoses, (2021).
- H. Foyaca-Sibat, L. Ibanez-Valdes, J. More-Rodriguez, Parasitic zoonoses of the brain: Another challenger?, J Neurol, 12(2009).
- E.P. David, L. SangJoon, D. Thirumala Kanneganti, PANoptosis in microbial infection, Curr Opin Microbiol, 59(2021):42-49.
- R.K.S. Malireddi, P. Gurung, S. Kesavardhana, Innate immune priming in the absence of TAK1 drives RIPK1 kinase activity-independent pyroptosis, apoptosis, necroptosis and inflammatory disease, J Exp Med, 217(2020).
- S. Chunxia, C. Pan, W. Yukun, Z. Qingqi, Z. Danmei, et al. PANoptosis: A cell death characterized by pyroptosis, apoptosis and necroptosis, J Inflamm Res, 16(2023):1523-1532.
- Y. Yang, L. Liu, I. Naik, Z. Braunstein, J. Zhong, et al. Transcription factor C/EBP homologous protein in health and diseases, Front Immunol, 8(2017):1612.
- J.M. Gullett, R.E. Tweedell, T.D. Kanneganti, It’s all in the PAN: Crosstalk, plasticity, redundancies, switches and interconnectedness encompassed by PANoptosis underlying the totality of cell death-associated biological effects, Cells, 11(2022):1495.
- R. Zhou, J. Ying, X. Qiu, A new cell death program regulated by toll-like receptor 9 through p38 mitogen-activated protein kinase signaling pathway in a neonatal rat model with sepsis associated encephalopathy, Chin Med J, 135(2022):1474-1485.
- C.Y. Taabazuing, M.C. Okondo, D.A. Bachovchin, Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages, Cell Chem Biol, 24(2017):507-514.
- S. Humberto Foyaca, Neurocysticercosis, epilepsy, COVID-19 and a novel hypothesis: Cases series and systematic review, Clin Schizophr Relat Psychoses, (2021).
- S. Humberto Foyaca, People living with HIV and neurocysticercosis presenting COVID-19: A systematic review and crosstalk proposals, Clin Schizophr Relat Psychoses, (2021).
- S. Humberto Foyaca, Comorbidity of neurocysticercosis, HIV, cerebellar atrophy and SARS-CoV-2: Case report and systematic review, Clin Schizophr Relat Psychoses, 15(2021).
- R.K.S. Malireddi, S. Kesavardhana, R. Karki, B. Kancharana, A.R. Burton, et al. RIPK1 distinctly regulates Yersinia-induced inflammatory cell death PANoptosis, Immunohorizons, 4(2020):789-796.
- H. Foyaca-Sibat, L. Ibanez-Valdes, Vascular dementia type Binswanger’s disease in patients with active neurocysticercosis, Rev Electron Biomed, 1(2003):32-42.
- X. Leyao, H. Huanshao, F. Shuhao, Z. Biying, W. Jianguo, et al. Ferroptosis: A mixed blessing for infectious diseases, Front Pharmacol, 13(2022):992734.
- C. Jiafeng, H. Lijuan, Y. Yue, X. Wei, Q. Qingchun, et al. Somatic cell reprogramming for nervous system diseases: Techniques, mechanisms, potential applications and challenges, Brain Sci, 13(2023):524.
- M. Faiz, N. Sachewsky, S. Gascon, K.W.Bang, C.M. Morshead, Adult neural stem cells from the subventricular zone give rise to reactive astrocytes in the cortex after stroke, Cell Stem Cell, 17(2015):624–634.
- T. Matsuda, T. Irie, S. Katsurabayashi, Y. Hayashi, T. Nagai, et al. Pioneer factor neurod1 rearranges transcriptional and epigenetic profiles to execute microglia-neuron conversion, Neuron, 101(2019):472–485.
- A. Dagenais, C. Villalba-Guerrero, M. Olivier, Trained immunity: A “new” weapon in the fight against infectious diseases, Front Immunol, 14(2023):1147476.
- M.G. Netea, J. Dominguez-Andres, L.B. Barreiro, T. Chavakis, M. Divangahi, et al. Defining trained immunity and its role in health and disease, Nat Rev Immunol, 20(2020):375-388.
- C. Heijden, M.P. Noz, L.A.B. Joosten, M.G. Netea, N.P. Riksen, et al. Epigenetics and trained immunity, Antioxid Redox Signal, 29(2018):1023–1040.
- H. Foyaca-Sibat, L. Ibanez-Valdes, The role of oxidative stress in neurocysticercosis: A comprehensive research, Clin Schizophr Relat Psychoses, 17(2023).
- M. Nico Garcia, H. Foyaca-Sibat, L. Ibanez-Valdes, Our hypotheses about the role of cuproferropanoptosis in neurocysticercosis and a comprehensive review, J Drug Alcohol Res, 12(2023).
- L. Yueying, C. Yuanjin, J. Xiaofan, M. Huiya, C. Yingsi, et al. Analysis of the role of PANoptosis in seizures via integrated bioinformatics analysis and experimental validation, Heliyon, 10(2024).
- H. Foyaca-Sibat, L. Ibanez-Valdes, New hypotheses on the role of microglias in ischemic reperfusion injury secondary to neurocysticercosis, Clin Schizophr Relat Psychoses, 17(2023).
- M. Ludmila, S.D. Benedetto, V. Muller, From homeostasis to neuroinflammation: Insights into cellular and molecular interactions and network dynamics, Cells, 14(2025):54.
- H. Chen, L. Wang, X. Zhou, L. Zou, X. Xiang, et al. Protective effects of Mdiviâ?1 on cognition disturbance following sepsis in mice via alleviating microglia activation and polarization, CNS Neurosci Ther, 31(2025):e70149.
- H. Ding, Y. Li, S. Chen, Fisetin ameliorates cognitive impairment by activating mitophagy and suppressing neuroinflammation in rats with sepsisâ?associated encephalopathy, CNS Neurosci Ther, 28(2022):247–258.
- L. Ying, G. Lei, G. Zhang, S. Wenjie, Y. Xiaohui, et al. Nogo-A exacerbates sepsis-associated encephalopathy by modulating microglial SHP-2/NLRP3 balance and inducing ROS and M1 polarization, Biomol Biomed, 25(2025):210-225.
Copyright: © 2025 Lourdes de Fatima Ibanez Valdes, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited