Research Article: Journal of Drug and Alcohol Research (2025) Volume 14, Issue 1
Opsoclonus Myoclonus Ataxia Syndrome Associates to Mixed Meningitis Responding to Drug Therapy: Case Report, New Hypotheses and Systematic Review
Lourdes de Fatima Ibanez Valdes and Humberto Foyaca Sibat*2Department of Neurology, Nelson Mandela Academic Hospital, Walter Sisulu University, South Africa
Humberto Foyaca Sibat, Department of Neurology, Nelson Mandela Academic Hospital, Walter Sisulu University, South Africa, Email: humbertofoyacasibat@gmail.com
Received: 14-Feb-2025, Manuscript No. JDAR-25-161336; Editor assigned: 18-Feb-2025, Pre QC No. JDAR-25-161336; Reviewed: 04-Mar-2025, QC No. JDAR-25-161336; Revised: 24-Mar-2025, Manuscript No. JDAR-25-161336; Published: 31-Mar-2025, DOI: 10.4303/JDAR/236427
Abstract
Introduction: Opsoclonus Myoclonus Ataxia Syndrome (OMAS), also called “dancing eye syndrome,” is an uncommon neurologic disorder typically presenting with both Myoclonus (Mc) and Opsoclonus (Oc) concurrently. We report a case presenting OMAS associated with mixed meningitis who recovered from the chaotic, involuntary, arrhythmic, multidirectional conjugate eye movements without intersaccadic intervals.
Methods: We perform a systematic search of the medical literature follows the guidelines suggested by PRISMA. From January 2023 to January 2025, the authors searched the scientific databases, Scopus, Embassy, Medline, PubMedCentral using the following searches: “opsoclonus” or “myoclonus” or “kinsbourne syndrome” or “Opsoclonus Myoclonus Ataxia Syndrome (OMAS)” or “cryptococcal meningitis” or “tuberculous meningitis” or “mixed meningitis” “paraneoplastic syndromes” or “pathogenesis of OMAS” or “treatment of OMAS”.
Results: After screening the full-text articles for relevance, 96 were included for final review. However, no article was selected when we searched for association/comorbidity with cryptococcal/TB meningitis.
Conclusions: As far we know this case is the first one presenting OMAS related to mixed meningitis. We delivered some hypotheses on the pathogenesis of this comorbidity based on the information reported by other investigators.
Keywords
Opsoclonus; Myoclonus; Ataxia; Cryptococcal infection; Dancing eyes syndrome; Kinsbourne syndrome; Thalamic opsoclonus; Cryptococcal meningitis; Tuberculous meningitis
Introduction
Opsoclonus Myoclonus Ataxia Syndrome (OMAS), also called “dancing eye syndrome,” is an uncommon neurologic disorder typically presenting with both Myoclonus (Mc) and Opsoclonus (Oc) concurrently. Oc is characterized by chaotic, involuntary, arrhythmic, multidirectional conjugate eye movements without intersaccadic intervals with a frequency of 10 Hz-25 Hz and 5-10 degrees of amplitude, worsening during random eyes movement or fixation, which remains present during eyelid closure or sleep and commonly associated to blurred vision, oscillopsia and vertigo. Oc differs from Ocular flutters (Of) in that (Of) the involuntary movements happen in the horizontal plane only, while in Oc, the unwanted saccade has vertical, horizontal and torsional components and always takes the eyes away from the target. The incidence of OMAS is estimated at approximately 1 per 5 million worldwide, 1.5-2 times higher in children between 1.5 and 2 years old. At the same time, adult presentation is mainly seen in patients ages 40 and 70 [1]. Para-infectious OMAS has a variety of viral and bacterial associations, including EBV, HSV, Salmonella, Streptococcus spp., CMV and West Nile Virus. At the same time, it has also been considered an autoimmune-mediated phenomenon [2,3]. Ri/Anti- Neuronal Nuclear Antibody type 2 (ANNA2) antibodies (henceforth called anti-Ri) are the neuronal antibodies most common to OMS. In contrast, anti-Yo/anti-Purkinje cell antibodies have been identified as a major contributor to the development of clinical cerebellar manifestations. On the other hand, natural killer and acetylcholine receptor antibodies have been found in cases presenting OMAS [4,5]. Moreover, more recently, Rosenow reported one patient presenting contactin-associated protein-like 2 (Caspr2)-positive OMAS in a setting of non-small cell lung carcinoma in a 46-year-old-woman characterized by without brain involvement intractable nausea and vomiting, vertigo, new onset gait instability, dysarthria, ataxia, myoclonus and opsoclonus. Caspr2 also plays a role in synaptogenesis, neurodevelopment and maintenance of synaptic integrity. Other authors reported patients presenting an angiosarcoma with OMS preceding clinical recurrence [6], falciparum malaria [7], associated with ovarian teratoma resection [8,9], associated with glutamic acid decarboxylase antibodies [10], associated with Mumps virus infection [11], Scrub typhus infection [12]. Panzer, et al. reported the presence of antibodies to dendritic neuronal surfaces in OMAS [13]. Other authors established that following herpes simplex infection and the para-infectious antibody-like anti-N-methyl-D-aspartate (receptor of seroconversion) can be linked to OMAS [14,15]. On the other hand, Almudhry and collaborators confirmed that the brain volume in 17 children presenting OMAS was smaller than that of normal healthy children. That group showed an abnormal growth trajectory (maturation) [16].
Regarding the humoral immunity in paraneoplastic OMAS have been established that the first indication of immune involvement was the presence of some antibodies such as anti-GABA2R, Anti-Ri, anti-Ma2, anti-CRMP5, anti-Hu, anti-Ma1, anti-GlyR, anti-NMDAR, anti-DPPX, Anti- LGI-1 [17] and anti-Kelch-like protein-11 antibodies [18]. The author revealed Myelin oligodendrocyte glycoproteinimmunoglobulin G positivity initiating an immune response in the postpartum period of the female patient presenting OMAS [19]. In 2021, Alburaiky and colleagues documented the increased type 1 interferon signalling to lead to neuroimmune dysregulation in patients with OMAS associated with Aicardi-Goutieres syndrome [20]. HIV infection, severe COVID-19, rare presentations of refractory paraneoplastic neurological syndrome such as large cell neuroendocrine carcinoma, which is a rare form of non-small cell lung cancer and even rhombencephalitis (inflammatory diseases of the pons, cerebellum and medulla oblongata) have been reported in association with OMAS as well [21-24].
Cryptococcal Meningitis (CM) is an infection caused by a fungus, leading to the Central Nervous System (CNS) inflammation. It is caused by Cryptococcus neoformans, a fungus mainly found in bird droppings and soil. There are two different types: Cryptococcus gattii and Cryptococcus neoformans, which are more commonly seen in HIVpositive patients.
Tuberculous Meningitis (TBM) caused by Mycobacterium tuberculosis can be spread to the CNS from another source in the body, usually the lung. TBM is the second cause of infectious vasculitis in our region, only preceded by HIV infection and followed by neurocysticercosis and neurosyphilis [25-31]. This study aims to report a case presenting an OMAS with associated cryptococcal infection of the brain, search the medical literature for other reported cases and based on information found in those publications, answer the following research question: What is the most likely pathogenesis of OMAS in those cases?
Materials and Methods
Search strategy
We perform a systematic search of the medical literature follows the guidelines suggested by PRISMA [32]. From January 2023 to January 2025, the authors searched the scientific databases, Scopus, Embassy, Medline, PubMed Central using the following searches: “Opsoclonus” or “myoclonus” or “kinsbourne syndrome” or “Opsoclonus Myoclonus Ataxia Syndrome (OMAS)” or “cryptococcal meningitis” or “tuberculous meningitis” or “mixed meningitis” “paraneoplastic syndromes” or “pathogenesis of OMAS” or “Treatment of OMAS”. After removing duplicates, two reviewers (LDFIV and HFS) from each side screened titles and abstracts and evaluated the full texts of eligible articles based on the proposed inclusion criteria. Any disagreement between the reviewers involved in the literature search was resolved through discussion with all authors to reach a consensus.
Selection criteria
The following manuscripts were included in the systematic review: Articles on OMAS, whether (OMAS-CM/TBM) or not, with detailed pathogenesis and/or drug therapy. Exclusion criteria were as follows: 1) Inaccessibility to full text; 2) Articles with unclear pathogenesis/drugs therapy of OMAS; 3) Lack of relevant clinicopathological data; 4) Non-original studies (i.e., editorials, letters, conference proceeding, book chapters); 5) Animal model studies; and 6) Non-/Spanish/Portuguese/English studies. The papers were thoroughly screened for duplicates.
Data extraction and quality assessment
All extracted data were collected in an excel electronic database, including pathogenesis, drug management, initial clinical presentation, evaluation of OMAS after treatment, follow-up and status at the latest evaluation. The quality of the studies included was categorized as poor, fair or reasonable, in agreement with the criteria of the National Institutes of Health (https://www.nhlbi.nih.gov/health-topics/studyquality-assessment-tools, (accessed on January 10, 2025). Two reviewers (LDFIV and HFS) independently evaluated the articles and the discussion resolved disagreements.
Statistical analysis
The primary objective of this study was to evaluate whether the pathogenesis/drug management of OMAS differs significantly among different ways of therapies. Without a comprehensive reference for the total number of OMAS cases, the prevalence of OMAS with associated CM/TBM was searched through a comprehensive review of the medical literature.
Calculating the proportion between OMAS/CM-TBM (from this systematic review) and overall number of cases of OMAS due to other causes, measured as the largest calculated sample size among the 10 most recent PRISMAbased systematic reviews published in the English literature before-cited in the introduction.
Statistical analyses were performed using XLSTAT (ad-on for Microsoft Excel, version 2021.4.1, Addinsoft SARL) and RStudio (version 4.3.1, https://www.rstudio.com/). Variations in continuous variables were assessed using the Mann-Whitney U-test. Fisher’s exact test or chi-square was used to evaluate the association among categorical variables, as appropriate. We presented descriptive statistics for continuous variables as median (95% Confidence Interval (95% CI)). The following time-to-event prognostic outcomes were analyzed: (1) Overall survival from cases presenting malignant tumours, considering the death of any cause as event and patients alive at the latest evaluation as censors; (2) Considering recurrence of OMAS and death of any cause as events and patients who were disease-free and alive at the latest evaluation as censors; (3) Time-torecurrence, considering recurrence of disease as event and diseaseâ?free patients (either alive or dead) as censors; (4) Diseas-specific survival, considering the death due to the disease as event and patients alive at the latest evaluation or dead of other cause as censors. All situations were evaluated using the method of Kaplan-Meier, to look for relevant prognosticators. A model of multivariable Cox proportional hazards with a priori selection of covariates was used to check for independent prognostic effects. To assess therapy’s protective effect, we built a multivariable model that included only OMAS cases. The results are expressed as p-value and hazard ratio with a 95% confidence interval. Statistical significance was defined at 0.05.
Results and Discussion
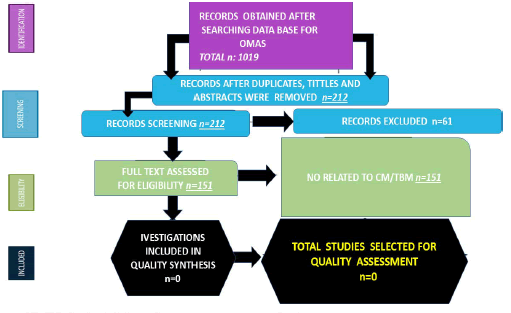
A total of 1019 titles were collected from the literature search. After removing duplicates and excluding 232 records, 807 relevant articles were examined. Four studies were unavailable for retrieving. After including six additional articles identified from citation searching, 61 were excluded for several reasons, 151. A total of 1019 records were identified from these searches. The resulting study titles were exported to excel and duplicates were removed, leaving 232 unique titles. We screened all remaining unique full-text articles, abstracts and titles for eligibility. Titles were typically excluded if they were unavailable in Spanish,Portuguese or English or irrelevant to OMAS. Articles relevant to OMAS, paraneoplastic syndromes, the clinical presentation, management, treatment, developmental consequences of OMAS, pathogenesis and treatment were included. A total of 212 articles remained following this initial screening. The authors then screened these articles by abstract. After further review, abstracts were excluded if they were irrelevant to the topic, outdated or unavailable in Spanish, Portuguese, English. With these reasons for exclusion, 61 abstracts were excluded, leaving 151 articles for full-text review. After screening the full-text articles for relevance, 96 were included for final review (Figure 1). No article was selected when we searched for association/ comorbidity with cryptococcal/TB meningitis.
Figure 1:PRISMA: Flow diagram with included publications (Source: Humberto, 2024)
Series description and differences among groups
All the studies selected before to be included had adequate relevance to the subject of this systematic review. None were randomized controlled trials or prospective studies; all the articles included were case reports and case series. Studies were published between 2004 and 2024.
The total number of patients presenting OMAS/CM/TBM was cero.
Median age was 17.5 (range 11-79) with significant differences between age groups (p<0.001). We did not find remarkable variations in gender (p=0.064), although females presenting OMAS were noticeably more frequent and slightly more prevalent (Tables 1 and 2).
| Patient's name: | MM | FILE NO. | 158441581 |
| Gender: | Male | DOA: | 27/12/2024 |
| Age: | 48 years old | DOD: | 21/01/2025 |
| Home language: | ISIXHOSA | ||
| Referring doctor: | DR M K | Referring hospital: | NMAH–INMED |
| Reason for referral: | Further MX | Potential risks: | N/A |
| Receiving doctor: | DR S J | ICD 10. CODE | |
| Procedures performed: | Final diagnosis: | ||
| N/A | OMAS and mixed meningitis-CCM+TBM young stroke | ||
Table 1: History of a patient.
| S. No | Drug name | Dose |
|---|---|---|
| 1 | Fluconazole 400 mg PO daily | X 1/12 |
| 2 | Daily | X 1/12 |
| 3 | Thiamine 100 mg PO daily | X 1/12 |
| 4 | Pyridoxine 50 mg PO DLY | X 1/12 |
| 5 | Folate 5 mg PO DLY | X 1/12 |
| 6 | VIT D 50000 IU monthly | X 1/12 |
| 7 | Calcium carbonate 500 mg DLY | X 1/12 |
| 8 | Simvastatin 20 mg PO NOCTE | X 1/12 |
| 9 | ASA 150 mg PO NOCTE | |
| 10 | Rifafour (TB tx) | X 1/12 |
Table 2: Treatment received.
A 48-year-old male municipal worker with nil comorbidities but a history of excessive ET-OH use presented with insomnia, opsoclonus, ataxia and other cerebellar signs.
Background history
The was last well till 22/12/2024, whereby pt was indulging in binge use of ET-OH of various kinds. 2/7 later, he developed insomnia, shaking of the eyes and head, especially when following an object and giddiness as well as loss of balance.
• He reported no dysphagia, both solids and liquids. There was no regurgitation.
• No changes in voice and speech-dysarthria.
• No loss of taste (Ageusia).
• No backache.
• No left-sided retrosternal pain.
• No chest tightness.
• No palpitations.
• No loss of weight.
• No night sweats (not typically drenching).
• No urinary incontinence.
• No intermittent headaches.
• No tremors of the hands.
• He denied any visual, olfactory or auditory losses.
Past medical history
PT came to NMAH–Neurology for thorough evaluation and management after review by internal medicine. He has never had any injuries or trauma previously. The family and patient deny the use of traditional medicine in his management. There is no surgical history of note. Nil known allergies.
Family history
No one else has any relevant medical history.
Social history
Pt works at the municipality and has had no issues.
Drug history: None of relevance.
Dietary history: Pt follows a mixed diet.
Exposure history: No evidence suggests any exposure to organic or inorganic irritants.
Review of systems
None of relevance.
Investigations:
• Blood workup: Full neuro panel including young stroke screening done.
• HIV negative.
• CLAT positive.
• Total Cholesterol-5.07.
• All the other tests-Grossly NAD.
• LP: Full neuro panel done.
• Opening pressure-10 cm H2O.
• Protein 7.23, repeat 4.5.
• Glucose 0.7, repeat 0.7.
• ADA 33.2, repeat 14.9.
• Erythrocytes 0, repeat 2.
• PMN 0, repeat 12.
• Lymph 0, repeat 4.
• CT: Showed right sides basal ganglia/ MCA territory old infarct.
• MRI: Done 20/01/2025-report pending.
• EMG/NCS: Not done.
All our esteemed colleagues from the allied services are assisting us in rehabilitating the patient. Based on the above presentation, our working differential diagnoses were:
• Opsoclonus Myoclonus Ataxia Syndrome (OMS).
• Young stroke due to infectious vasculitis.
• Mixed Meningitis-cryptococcal meningitis and TB meningitis in an HIV uninfected patient.
With the initial assumption of OMS in mind, we started the pt on Methylprednisolone 1g IVI daily × 3/7, then observed for improvement, but there was none.
Then, once the LP results were available and we confirmed the results, we initiated.
1. Amphotericin B to which he showed an allergic reaction and it was stopped.
2. Fluconazole 800 mg po daily.
• He has completed 2/52 so far.
• PLAN is to titrate it to 400 mg daily for 2/12.
• Then, a maintenance dose of 200 mg daily for about 10/12, with a total treatment duration of 1 year.
3. Flucytosine-unavailable at NMAH.
4. RHZE 5 tabs daily was also initiated for 2/12.
• To be converted to RH 150/300 mg 2 tabs daily from 8/12 to 10/12.
5. Neurosupplements–already initiated viz.,
• Thiamine 100 mg daily,
• Pyridoxine 50 mg daily,
• Folate 5 mg daily,
• Vit D 50000 IU every 2 weeks,
• Ca carbonate 500 mg daily.
6. For the stroke and high cholesterol
• Simvastatin 20 mg nocte.
• ASA 150 mg nocte.
7. Aggressive physical therapy.
Based on the clinical presentation, the lab results and the rapid response that the patient showed once initiated on fluconazole and RHZE, we conclude that the working diagnosis is mixed meningitis–TBM and CCM as well as previous young stroke.
Comments and concluding remarks
Pathogenesis: Opsoclonus’ pathogenesis remains unknown. The two hypotheses are the cerebellar and brainstem theories [33]. Typically, saccades require the involvement of the saccadic system, which is in the brainstem with the participation of the cerebellum. The system includes both burst Neuron Cells (NC) and omnipause NC. The expression of burst NC projects onto extraocular ocular motoneurons located in the oculomotor, trochlear and abducens nucleus, producing saccades to be generated. As mentioned, it also receives projections from the vermis of the cerebellum, specifically from the Fastigial Nuclei (FN). Omnipause neurons oversee preventing any unwanted saccades from being made in any direction. Omnipause NC gets NC projections from the mesencephalic reticular formation, the Superior Colliculus (SC) and other areas. Abnormal eye movements indicate saccadic oscillations. Other investigators have documented that disorders affecting the saccadic system and superior regulatory centres could theoretically cause saccadic oscillations [34].
Nevertheless, the elevation of the burst neuron excitability refers to a condition where sure NC in the brain are firing in rapid bursts more fasting than the standard rate, leading to abnormal brain activity associated with some neurological diseases such as epilepsy, Parkinson’s disease or even some mental issues, where the heightened firing can disrupt regular network activity; essentially, the neurons are becoming hyper excited and firing in rapid bursts instead of keeping a regular controlled firing pattern. Based on brainstem theory, opsoclonus is caused by increased burst neuron excitability and diminished omnipause neuron inhibition [35]. According to Chaumont, et al., COVID-19-associated abnormal eye movement is due to brainstem disorder [36]. Based on the information released by other authors [37], we hypothesized that omnipause NC is modulated by some excitatory neurotransmitter such as glycine, while anti- GlyR antibodies might change the inhibitory properties of saccadic burst NC, as is shown in Figure 2.
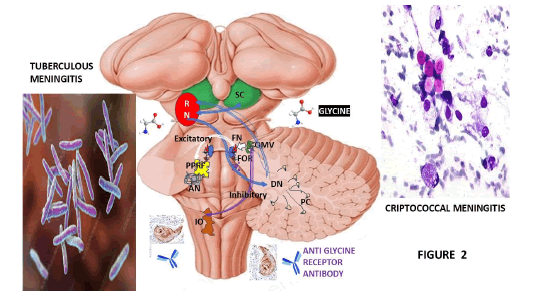
Figure 2: Graphical representation of the elements involved in the pathogenesis of OMAS due to CM/TBM/IV. Note: AN: Abducens Nerve; DN: Dentate Nucleus; FOR: Fastigial Oculomotor Region; IO: Inferior Olive; OMV: Ocular Motor Vermis; PPRF: Para Pontine Reticular Formation; RN: Red Nucleus
The FN is located at the vermis of the cerebellum bilaterally and it projects to various regions, including the medial reticular formation, vestibular nuclei and cervical cord, playing a role in eye movements and motor coordination. The FN disinhibition causes saccadic oscillation. Functional MRI evidence demonstrated bilateral activation of the FN [37]. In some cases of OMAS, autopsies have long revealed demyelination in the cerebellum with loss of Purkinje cells and electrophysiology studies have confirmed that opsoclonus-myoclonus originates in the brainstem, with simultaneous abnormalities in the cerebellar network [38].
We hypothesize that in our case, the direct damage caused by TBM/CM and its associated Infectious Vasculitis (IV) can affect the generation of saccades toward the opposite side (contralateral saccades) facilitated by the Fastigial Oculomotor Region (FOR) on each side of the cerebellum, which also supports the termination of saccades toward the same side (ipsilateral saccades), as represented in Figure 2.
This mechanism happens via projections from the FOR to the omnipause NC and inhibitory/excitatory burst NC in the midbrain/pons/medulla oblongata but also becomes dysfunctional due to the before cited inflammatory response to the bacterial/fungal infection; it also affects the normal pathway of the axons arising from the FN decussate within the cerebellum which terminates primarily in the contralateral vestibular or premotor brainstem nuclei. As we speculate, the right FOR increases its activity before the initial leftward saccades and toward the end of the rightward saccades. Therefore, mixed meningitis disturbs the function in one FOR and may cause saccade hypermetria toward the ipsilateral side and hypometria toward the contralateral side. We also hypothesize that a unilateral structural FOR lesion never happens because the FOR output crosses immediately and runs through the opposite FOR before exiting the cerebellum through the superior cerebellar peduncle; still, in our case, infectious meningitis affects all regions of the cerebellum/brainstem. At the same time, the dorsal Oculomotor Vermis (OMV) behaves in a similar but reciprocal way because it has an inhibitory effect on the FOR. Therefore, the pattern of saccade dysmetria with damage caused by CM/TBM/IV in the OMV is in the contralateral FOR.
Saccade hypometria is toward the same side as the lesion, while hypermetria is toward the contralateral side of the lesion. We hypothesized that with the additional involvement of the SC, eye movements become chaotic in all directions while the damage persists [39].
We also hypothesized that CM/TBM/IV of the OMV and its underlying FOR may have the same functional contribution to modulate pursuit as for the saccades. Therefore, CM/ TBM/IV lesions of the FOR will impair pursuit away from the lesion’s ipsilateral side but enhance contralateral pursuit acceleration. We speculated that CM/TBM/IV injuries of the OMV lead to pursuit being impaired toward the ipsilateral predominant side of the lesion, while the smooth pursuit deficit is mainly at onset and termination. In contrast, the archicerebellum is probably more concerned with pursuit during sustained tracking, also affecting the posture and balance secondary to archicerebellum involvement [39].
In our hypotheses on the pathogenesis of saccadic hypermetria/hypometria related to lesions caused by CM/TBM/IV in the FOR/OMV, we highly recommend considering the role of cerebellar control of saccades which can be well understood if one considers its neuroanatomical circuitry: As well documented during the lectures by NASA Courses for doctors, the OMV receives information about performance during saccades and adjusts its disinhibition upon the FOR to ensure the saccade arrives on target. These authors explained that the OMV is also important for saccade adaptation and the plasticity for this motor learning happens at the level of its Purkinje cells, based on an error signal which arises in the SC, as we considered in Figure 2.
In Figure 2, we represented the Paramedian Pontine Reticular Formation (PPRF) because it contains excitatory burst neurons and caudal to it, there is a portion of the medullary reticular formation that contains Inhibitory Burst Neurons (IBN), which project to the contralateral abducens nucleus for horizontal saccade. At the same time, it connects to the trochlear and oculomotor nucleus at the midbrain through the medial longitudinal fasciculus for horizontal eye movements. The lesions caused by CM/ TBM/IV lead to opsoclonus with the participation of the cerebellum, SC and the uncinate fasciculus, which send excitatory projections from the contralateral FN to the pontine and medullary reticular formations. This pathway passes through the midline and bends around the Superior Cerebellar Peduncle (RSCP) to the midbrain [39].
We hypothesized that a focal ischemic lesion due to IV of the left FN or direct lesion due to CM/TBM of the underlying deep cerebellar white matter (hook bundle) in the region of the RSCP would result in decreased excitation of EBNs and IBNs on the right leading to hypometria of rightward saccades. The FN modulate the deacceleration of ipsilateral saccades. Similarly, to the antagonist burst of agonist-antagonist muscle activation, cells in the left FN will fire before the end of leftward saccades and by exciting IBNs on the right, will cause inhibition of abducens motor neurons on the left side. We also consider that CM/TBM/ IV leads to a failure to produce this inhibitory action, which might result in a prolonged pulse applied to the left abducens motor neurons, consequently producing hypermetric saccades. If CM/TBM/IV causes a hook bundle to the lesion, the hypermetric saccades are seen, away from the side of the lesion, associated with hypermetric saccades towards the side of the lesion, which is also known as contraption and as has been reported in cases with multiple sclerosis [39]. We also believe an OMV lesion caused by CM/TBM/IV would stop cerebellar cortical inhibition of the deep cerebellar nuclei, including the FOR, resulting in FOR overactivity.
In patients presenting CM/TBM/IV lesions of the Restiform Body (RB), we must consider that the NC at the FN receives inhibitory projections from PC of the same side of the cerebellar vermis. Those PC receive climbing fibre input from the opposite inferior olive via the restiform body and mossy fibre input via the middle cerebellar peduncle. When a climbing fibre excites a PC, there is a refractory period with decreased PC inhibitory expression on fastigial cells. Therefore, an ischemic lesion due to IV of the restiform body will cause loss of climbing fibre input and fewer PC refractory periods. It leads to a net increment of PC inhibition of fastigial neurons and functional inhibition of the FOR ipsilaterally. Nonetheless, an ischemic lesion due to IV on the left restiform body will cause increased inhibition of the left fastigial nucleus (FOR), leading to ipsipulsion with hypermetric saccades to the left, towards the ipsilateral side of the lesion and hypometric saccades to the contralateral side.
The pathogenesis of OMAS related to malignant tumour will be related to the types of autoantibodies released from each one. The most reported malignant tumour associated with OMAS in adults is the small-cell carcinoma of the lung. However, other patients presenting melanoma, breast cancer, urogenital and gynaecologic cancers have been published [40-47] and all pathogenic mechanisms will show some differences. This statement also includes those cases presenting OMAS due to SARS-CoV-2 [48]. Finsterer and Vasudevan found 45 patients with SARSCoV- 2 associated OMS/OMAS reported in the medical literature, whose ages ranged from 2 to 88 years. Our patient responded exceptionally well to the CM/TBM/IV and almost all clinical manifestations belonging to OMAS disappeared.
Brief comments on drug therapy for OMAS
Zhang and collaborators [33] established that this autoimmune neurological disorder mediated by humoral and cellular immunity can respond very well to combined immunotherapy. The incidence of recurrence and refractory presentations is expected to decrease. They also documented that the first step for all cases presenting with OMAS is a thorough diagnostic assessment looking for an underlying tumour because its incidence is high and because after removing the tumour, many patients improve. Therefore, all patients should have CT scans of the abdomen, chest and pelvis, brain magnetic resonance imaging and positron emission tomography. Notwithstanding, if these investigations came back negative, all of them should be repeated every four months for the next one and half years before being considered OMAS as an idiopathic one because some neoplasms can manifest later than the clinical features. Although increased neopterin in CSF does not have the sensitivity to be a biomarker, it can support T-cell activation and cell-mediated immunity in OMS [49]. We considered it should be taken into consideration at the time of planning a drug therapy because the success of B-cell-based immunotherapies and the discovery of CSF lymphocytic pleocytosis support the contribution of immune cells to OMAS immunopathogenesis as has been proposed by other authors [49].
The same authors have proved that immunosuppression strategies must include glucocorticoids, such as dexamethasone or Intravenous (IV) methylprednisolone. In contrast, immunomodulation choices include Intravenous Immune Globulin (IVIG), oral dexamethasone, cyclophosphamide and rituximab [33]. As hypothesized, immunopathogenesis, the expression of the immune system induced by immune cells, will include drug therapy as follows: 1) Prevention of immune activation, 2) Limiting T/B cell production and accelerating T/B cell removal. The most common drugs are corticosteroids, Adrenocorticotropic Hormone (ACTH) and Intravenous Immunoglobulins (IVIG) [50,51]. Other investigators also confirmed that the drug therapy of OMAS includes corticosteroids, IVIG and immunomodulating agents, such as Rituximab (Rx), cyclophosphamide, azathioprine, anti-neoplastic and in some anecdotic situations, antiepileptic drugs and even anxiolytics medications [52]. Rx (300 mg/m2) is an anti- CD20 monoclonal antibody that inhibits B cell expansion (so patients with normal B cell counts require rituximab) and is generally prescribed as broad-acting immunosuppressive agent for second-line treatment of reoccurring and refractory OMAS [53,54] which has the advantage of shortening the time of steroid use with a better prognosis by lowering the risk of disability [55]. Rx has been used as an adjunct therapy to steroids or ACTH and B-cell depletion in the CSF has been well documented by many authors [56-58]. It has also been prescribed to treat relapse [59]. Unfortunately, Rx increases the risk of sepsis and tumour growth [60]. On the other hand, patients presenting OMAS related to neoplasm must begin with tumour resection because it can help alleviate some of the symptoms [61]. Baclofen (45 mg/day), clonazepam (3 mg/day) and levetiracetam (3000 mg/day) are also prescribed as symptomatic treatments associated with immunotherapy. Clonazepam is prescribed for patients who have significant severe sleep disturbances with opsoclonus [62]. Topiramate has also shown good results as well [63]. To better manage myoclonus, zonisamide (300 mg/d to 500 mg/d divided into 1 or 2 daily doses) and anticholinergics drugs can be added [64].
Antimicrobial therapy and immunotherapies reduce the mortality rate of patients presenting OMAS due to herpes simplex virus [33]. Therefore, we hypothesized that empiric antiviral therapy, such as ganciclovir therapy, is strongly indicated in patients with a high suspicion of viral infection. Many therapy drug modalities have been used for OMAS, with no specific treatment showing reliably consistent 100% resolution of all clinical manifestations [65]. Notwithstanding, early drug therapy is indicated and is associated with better long-term outcomes [66]. Mizia-Malarz and collaborators reported that long-term sequelae occurred in all cases that received drug therapy in the late stage and only 42% of patients were treated at the beginning [67]. A randomized controlled clinical trial of 53 patients demonstrated a remarkable superior response in cases where a combination of prednisolone and IVIG was given, compared to prednisolone alone [68]. In patients who are not responding to the more conventional therapies, plasmapheresis has been performed to treat OMAS and it has proven to be successful, as it removes autoantibodies from the blood circulation [69-71]. Therapy using autologous cell transplantation has been used in two patients, showing a complete resolution in one of them while the other one showed minimal improvement. Therefore, removing pathogenic autoreactive cellular elements and replacing them with normal immune cells helps to improve OMAS [72]. Another therapeutic choice to treat cases of OMAS is ofatumumab [73,74], which has produced some spontaneous resolution of OMAS symptoms followed by normal growth and development without any immunotherapies [75]. Combining or multimodal therapies are typically used in treating OMAS with better outcomes [76].
Some researchers compared the efficacy and safety of combination drug therapies and found that dexamethasonebased combination immunotherapies or corticotropin have better efficacy than conventional monotherapy using dexamethasone or corticotropin alone [77,78].
This manuscript comprises one of the five reported patients [79-82] regarding anaesthesia management in OMAS. It is the first report to describe a successful combined neuraxialgeneral approach.
Conclusion
Unfortunately, there is no confirmed confident drug therapy for patients presenting OMAS due to side effects of SARS-CoV-2 vaccination. We believe that due to the scanty consensus related to how better to manage OMAS, a variety of drug therapies may need to be trialled to address the best response and outcome effectively.
Limitations of the Study
The present study has several limitations that are worthy of mention:
Case reports: The review primarily included case reports, which can limit the generalizability of findings due to variability and heterogeneity.
Staging: The Kadish-INSICA staging is intended for OMAS, which limits its utility since most cases are, by definition, secondary to malignant tumours/autoimmune processes. It might not adequately represent the complexity of paraneoplastic syndrome extension.
Prevalence: Comparing the prevalence of OMAS across different aetiological groups, including those with different sample sizes and inclusion methodologies, involves a degree of extrapolation and potentially forced alignment. This approximation introduces a potential assumption bias.
Missing data: Some older articles lacked details relevant to the study, which excluded some information from the analyses.
Evolution of treatment: Over the last decades, surgical techniques for removing primary tumours and managing sinonasal cancers may have improved outcomes, potentially affecting the comparability of older data with more recent data.
Unknown margin status: Several studies did not provide information on margin status, which prevented the inclusion of this relevant information in the prognostic analysis.
Author Contributions
Both investigators have read and agreed to the published version of the manuscript.
Conflict of Interest Statement
The authors declare they have no conflicts of interest.
Funding Information
The authors received no funds to perform the present research.
Ethics Statement and Consent
The study was conducted using the principles of the Helsinki Declaration, the Italian and US privacy and sensitive data laws and the internal regulations for retrospective studies of the Otolaryngology Section at Padova University and Brescia University (Italy).
Informed Consent Statement
We obtained the informed consent from the case involved in the study.
Data Availability Statement
The corresponding author will make the raw data supporting this article’s conclusions available upon request.
Acknowledgments
The authors thank Dr Sibi Joseph for his participation in the management of this patient the summary of the case presentation.
References
- C.S. Rosenow, S. Dawit, L.P. Farrugia, Henry, M.A KA, et al. Case report: Opsoclonus-myoclonus syndrome associated with contactin-associated protein-like 2 and acetylcholine receptor autoantibodies in the setting of non-small cell lung carcinoma, Neurohospitalist, 12(2022):100-104.
[Crossref] [Google Scholar] [PubMed]
- J.P. Klaas, J.E. Ahlskog, S.J. Pittock, J.Y. Matsumoto, A.J. Aksamit, et al. Adult-onset opsoclonus-myoclonus syndrome, Arch Neurol, 69(2012):1598-1607.
[Crossref] [Google Scholar] [PubMed]
- G. Berridge, D.A. Menassa, T. Moloney, P.J. Waters, I. Welding, et al. Glutamate receptor δ2 serum antibodies in pediatric opsoclonus myoclonus ataxia syndrome, Neurology, 91(2018):714-723.
[Crossref] [Google Scholar] [PubMed]
- T. Armangué, L. Sabater, E. Torres-Vega, E. Martínez-Hernández, H. Arino, et al. Clinical and immunological features of opsoclonus-myoclonus syndrome in the era of neuronal cell surface antibodies, JAMA Neurol, 73(2016):417-424.
[Crossref] [Google Scholar] [PubMed]
- J.R. Galli, S.L. Clardy, M.M Soldan, Adult-onset opsoclonus-myoclonus syndrome associated with ganglionic acetylcholine receptor autoantibody, Neurologist, 21(2016):99-100.
[Crossref] [Google Scholar] [PubMed]
- K. Periasamy, N. Das, D. Khosla, R. Kapoor, Recurrent angiosarcoma of scalp with opsoclonus myoclonus syndrome: Role of salvage treatment, BMJ Case Rep, 14(2021):241824.
[Crossref] [Google Scholar] [PubMed]
- K. Bose, S. Saha, MR. Islam, C. Chakraborty, M. Laskar, Opsoclonus myoclonus ataxia syndrome due to falciparum malaria in two Indian children, Indian J Ophthalmol, 64(2016):852-854.
[Crossref] [Google Scholar] [PubMed]
- A.A. Jones, T. Chen, Delayed opsoclonus–myoclonus syndrome after ovarian teratoma resection, J Neuroophthalmol, 42(2022):450-451.
[Crossref] [Google Scholar] [PubMed]
- K.V. Mahesh, R. Bansal, D. Naheed, N. Tandyala, R. Singh, et al. Opsoclonus myoclonus syndrome due to an ovarian teratoma: A case report and review of literature, Neuro-Ophthalmol, 44(2020):258-261.
[Crossref] [Google Scholar] [PubMed]
- H.S. Bhandari, Presentation of opsoclonus myoclonus ataxia syndrome with glutamic acid decarboxylase antibodies, BMJ Case Rep, 2012.
[Crossref] [Google Scholar] [PubMed]
- B.H. Kang, J.I. Kim, Opsoclonus-myoclonus syndrome associated with mumps virus infection, J Clin Neurol, 10(2014):272.
[Crossref] [Google Scholar] [PubMed]
- A. Neela, R. Gohil, R. Tagore, T.A. Vidya, R.M. Gohil, et al. Opsoclonus: A rare neurological manifestation in a patient with scrub typhus infection, Cureus, 16(2024).
- J.A. Panzer, R. Anand, J. Dalmau, D.R. Lynch, Antibodies to dendritic neuronal surface antigens in opsoclonus myoclonus ataxia syndrome, J Neuroimmunol, 286(2015):86-92.
[Crossref] [Google Scholar] [PubMed]
- J.P. Stahl, A. Mailles, Herpes simplex virus encephalitis update, Curr Opin Infect Dis, 32(2019):239-243.
[Crossref] [Google Scholar] [PubMed]
- T. Armangue, F. Leypoldt, I. Málaga, M. Raspallâ?Chaure, I. Marti, et al. Herpes simplex virus encephalitis is a trigger of brain autoimmunity, Ann Neurol, 75(2014):317-323.
[Crossref] [Google Scholar] [PubMed]
- M. Almudhry, M.W. Wagner, G. Longoni, C. Yea, L. Vidarsson, et al. Brain volumes in opsoclonus-myoclonus ataxia syndrome: A longitudinal study, J Child Neurol, 39(2024):129-134.
[Crossref] [Google Scholar] [PubMed]
- D. Smyth, K.M. Kyaw, A. Legister, G. MacFarlane, U.U. Sankar, et al. Post-COVID-19 opsoclonus-myoclonus syndrome and encephalopathy associated with Leucine-Rich Glioma-Inactivated 1 (LGI-1) antibodies, J Neurol Sci, 430(2021):119982.
[Crossref] [Google Scholar] [PubMed]
- E. Fonseca, R. Varas, J. Godoy-Santín, R. Valenzuela, P. Sandoval, Opsoclonus-myoclonus syndrome associated with anti Kelch-like protein-11 antibodies in a young female patient without cancer, J Neuroimmunol, 355(2021):577570.
[Crossref] [Google Scholar] [PubMed]
- S. Adhikari, A. Thuringer, L. Maali, Y. Jassam, Opsoclonus myoclonus syndrome in a postpartum period, Mult Scler Relat Disord, 50(2021):102862.
[Crossref] [Google Scholar] [PubMed]
- S. Alburaiky, R.C. Dale, Y.J. Crow, H.F. Jones, E. Wassmer, et al. Opsoclonusâ?myoclonus in Aicardiâ?Goutières syndrome, Dev Med Child Neurol, 63(2021):1483-1486.
[Crossref] [Google Scholar] [PubMed]
- N.M. Pereira, I. Shah, S. Kulkarni, Opsoclonus–myoclonus–ataxia syndrome in an HIV-infected child, Oxford Med Case Rep, 2016(10):077.
- H. Chaumont, A. San-Galli, F. Martino, C. Couratier, G. Joguet, et al. Mixed central and peripheral nervous system disorders in severe SARS-CoV-2 infection, J Neurol, 267(2020):3121-3127.
[Crossref] [Google Scholar] [PubMed]
- T. Saito, A. Maeda, H. Nagano, T. Kishaba, T. Kishaba, A case of paraneoplastic neurological syndrome leading to the diagnosis of large cell neuroendocrine carcinoma from opsoclonus-myoclonus syndrome, Cureus, 15(2023).
[Crossref] [Google Scholar] [PubMed]
- B. Jubelt, C. Mihai, T.M. Li, P. Veerapaneni, Rhombencephalitis/brainstem encephalitis, Curr Neurol Neurosci Rep, 11(2011):543-552.
[Crossref] [Google Scholar] [PubMed]
- L. de Fatima Ibanez Valdes, H.F. Sibat, New hypotheses on the role of microglias in ischemic reperfusion injury secondary to neurocysticercosis, Clin Schizophr Relat Psychoses, 17(2023).
- L. de Fatima Ibanez Valdes, The role of rouget cells in neurocysticercosis. J Drug Alcohol Res, 13(2024):17.
- T. Mongezi, J. Sibi, G. Jerry, L. de Fátima IV, D. Tozama, et al. Atypical HIV-vacuolar myelopathy: A case report, Eur J Med Res, 26(2021):1-6.
[Crossref] [Google Scholar] [PubMed]
- L. de Fatima Ibanez Valdes, H.F. Sibat, Obstructive sleep apnea syndrome and neurocysticercosis, J Drug Alcohol Res, 13(2024).
- L. de Fatima Ibanez Valdes, H.F. Sibat, The oxidative stress in neurocysticercosis, J Drug Alcohol Res, 13(2024):15.
- L. de Fatima Ibanez Valdes, H.F. Sibat, Comorbidity of alcohol use disorder and neurocysticercosis aggravated by dysbiosis, J Drug Alcohol Res, 13(2024).
- L. de Fatima Ibanez Valdes, H.F. Sibat, Ferropanoptosis in neurocysticercosis: Implications for novel therapeutic drug developmentâ?a comprehensive review, J Drug Alcohol Res, 13(2024):10.
- M.J. Page, J.E. McKenzie, P.M. Bossuyt, I. Boutron, T.C. Hoffmann, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews, BMJ, 17(2021):372.
[Crossref] [Google Scholar] [PubMed]
- X. Zhang, W. Yan, Y. Song, H. Zhu, Y. Sun, Adult-onset idiopathic opsoclonus-myoclonus syndrome, Brazilian Arch Ophthalmol, 87(2023):2022-2024.
[Crossref] [Google Scholar] [PubMed]
- D.S. Zee, D.A. Robinson, A hypothetical explanation of saccadic oscillations, Ann Neurol, 5(1979):405-414.
[Crossref] [Google Scholar] [PubMed]
- S. Ramat, R.J. Leigh, D.S. Zee, L.M. Optican, Ocular oscillations generated by coupling of brainstem excitatory and inhibitory saccadic burst neurons, Exp Brain Res, 160(2005):89-106.
[Crossref] [Google Scholar] [PubMed]
- H. Chaumont, A. San-Galli, F. Martino, C. Couratier, G. Joguet, et al. Mixed central and peripheral nervous system disorders in severe SARS-CoV-2 infection, J Neurol, 267(2020):3121-3127.
[Crossref] [Google Scholar] [PubMed]
- C. Helmchen, H. Rambold, A. Sprenger, C. Erdmann, F. Binkofski, Cerebellar activation in opsoclonus: An fMRI study, Neurology, 61(2003):412-415.
[Crossref] [Google Scholar] [PubMed]
- K.A. Gwinn, J.N. Caviness, Electrophysiological observations in idiopathic opsoclonus–myoclonus syndrome, Mov Disord, 12(1997):438-442.
[Crossref] [Google Scholar] [PubMed]
- E.M. Frohman, T.C. Frohman, J. Fleckenstein, M.K. Racke, K. Hawker, et al. Ocular contrapulsion in multiple sclerosis: Clinical features and pathophysiological mechanisms, J Neurol Neurosurg Psychiatry, 70(2001):688-692.
[Crossref] [Google Scholar] [PubMed]
- A. Alkan, U. Cenikli, S. UylaÅ?, M. Yılmaz, T. Çakır, et al. Treatment-refractory paraneoplastic opsoclonus–myoclonus syndrome in a patient with small-cell carcinoma of the lung, J Oncol Pharm Pract, 26(2020):209-211.
[Crossref] [Google Scholar] [PubMed]
- S. Laroumagne, X. Elharrar, B. Coiffard, J. Plojoux, H. Dutau, et al. Dancing eye syndrome secondary to opsoclonusâ?myoclonus syndrome in smallâ?cell lung cancer, Case Rep Med, 1(2014):545490.
[Crossref] [Google Scholar] [PubMed]
- K.T. Stewart, J.S. Lee, G. Stuart, Paraneoplastic opsoclonus-myoclonus syndrome as a presentation of high grade serous ovarian cancer, Gynecol Oncol Rep, 30(2019):100511.
[Crossref] [Google Scholar] [PubMed]
- L. Martins, D. Galvão, A. Silva, B. Vieira, Ó. Reis, et al. Paraneoplastic opsoclonus–myoclonus syndrome as a rare presentation of breast cancer, J Surg Case Rep, 2(2019):365.
[Crossref] [Google Scholar] [PubMed]
- A. Kostoglou, D. Vlastos, A. Bakalis, D. Ghosh, Breast cancer-associated opsoclonus-myoclonus syndrome: A case report, World J Surg Oncol, 19(2021):1-5.
[Crossref] [Google Scholar] [PubMed]
- J.D. Mondragón, O. Jiménez-Zarazúa, L.N. Vélez-Ramírez, M.A. Martínez-Rivera, S. Enríquez-Maciel, et al. Paraneoplastic opsoclonus-myoclonus syndrome secondary to melanoma metastasis form occult primary cancer, Case Rep Neurol, 11(2019):66-79.
[Crossref] [Google Scholar] [PubMed]
- C.J. Prestigiacomo, C. Balmaceda, J. Dalmau, Antiâ?Ri–associated paraneoplastic opsoclonusâ?ataxia syndrome in a man with transitional cell carcinoma: A case report, Int J Cancer Res, 91(2001):1423-1428.
[Crossref] [Google Scholar] [PubMed]
- S. de Luca, C. Terrone, S. Crivellaro, A. de Zan, P. Polo, et al. Opsoclonus-myoclonus syndrome as a paraneoplastic manifestation of renal cell carcinoma: A case report and review of the literature, Urol Int, 68(2002):206-208.
[Crossref] [Google Scholar] [PubMed]
- J. Finsterer, F.A. Scorza, Opsoclonus myoclonus ataxia syndrome due to SARS-Cov-2, Neuro-Ophthalmol, 47(2023):1-6.
[Crossref] [Google Scholar] [PubMed]
- M.R. Pranzatelli, K. Hyland, E.D. Tate, L.A. Arnold, T.J. Allison, et al. Evidence of cellular immune activation in children with opsoclonus-myoclonus: Cerebrospinal fluid neopterin, J Child Neurol, 19(2004):919-924.
[Crossref] [Google Scholar] [PubMed]
- J. Stiefel, E. Basu, R. Meyer, G. Kaur, Y. Khakoo, An unusual case of opsoclonusâ?myoclonusâ?ataxia syndrome associated neuroblastoma: Highâ?risk disease requiring immunotherapy, Pediatr Blood Cancer, 67(2020):28393.
[Crossref] [Google Scholar] [PubMed]
- M. Hsu, I. Tejani, N. Shah, R. Olaosebikan, A. Kumar, et al. Review of opsoclonus-myoclonus ataxia syndrome in pediatric patients, Children, 11(2024):367.
[Crossref] [Google Scholar] [PubMed]
- A. Borker, N. Choudhary, Rituximab, Indian Pediat, 48(2011):627-632.
- M.R. Pranzatelli, E.D. Tate, A.L. Travelstead, C.A. Baumgardner, N.V. Gowda, et al. Insights on chronic-relapsing opsoclonus-myoclonus from a pilot study of mycophenolate mofetil, J Child Neurol, 24(2009):316-322.
[Crossref] [Google Scholar] [PubMed]
- M.R. Pranzatelli, E.D. Tate, A.L. Travelstead, J. Barbosa, R.A. Bergamini, et al. Rituximab (anti-CD20) adjunctive therapy for opsoclonus-myoclonus syndrome, Pediatr Hematol Oncol, 28(2006):585-593.
[Crossref] [Google Scholar] [PubMed]
- M.R. Pranzatelli, E.D. Tate, J.A. Swan, A.L. Travelstead, J.A. Colliver, et al. B cell depletion therapy for newâ?onset opsoclonusâ?myoclonus, Mov Disord, 25(2010):238-242.
[Crossref] [Google Scholar] [PubMed]
- M.R. Pranzatelli, E.D. Tate, N.R. McGee, C.A. MacArthur, Evaluation of responsiveness to reduced-dose rituximab in corticotropin/intravenous immunoglobulin/rituximab combination immunotherapy for opsoclonus-myoclonus syndrome, Pediatr Neurol, 85(2018):71-75.
[Crossref] [Google Scholar] [PubMed]
- D. Toyoshima, N. Morisada, Y. Takami, H. Kidokoro, M. Nishiyama, et al. Rituximab treatment for relapsed opsoclonus–myoclonus syndrome, Brain Dev, 38(2016):346-349.
[Crossref] [Google Scholar] [PubMed]
- B.H. Chang, T. Koch, K. Hopkins, S. Malempati, Neuroblastoma found in a 4-year-old after rituximab therapy for opsoclonus-myoclonus, Pediatric Neurol, 35(2006):213-215.
[Crossref] [Google Scholar] [PubMed]
- T. Armangué, L. Sabater, E. Torres-Vega, E. Martínez-Hernández, H. Ariño, et al. Clinical and immunological features of opsoclonus-myoclonus syndrome in the era of neuronal cell surface antibodies, JAMA Neurol, 73(2016):417-424.
[Crossref] [Google Scholar] [PubMed]
- A.B. Pena, J.N. Caviness, Physiology-based treatment of myoclonus, Neurotherapeutics, 17(2020):1665-1680.
[Crossref] [Google Scholar] [PubMed]
- Y. Degirmenci, H. Kececi, Topiramate response in adult-onset opsoclonus-myoclonus-ataxia syndrome: A case report, Revue Neurol, 173(2017):418-420.
[Crossref] [Google Scholar] [PubMed]
- S. Chokroverty, M.K. Manocha, R.C. Duvoisin, A physiologic and pharmacologic study in anticholinergicâ?responsive essential myoclonus, Neurology, 37(1987):608.
[Crossref] [Google Scholar] [PubMed]
- M. Hsu, I. Tejani, N. Shah, R. Olaosebikan, A. Kumar, et al. Review of opsoclonus-myoclonus ataxia syndrome in pediatric patients, Children, 11(2024):367.
[Crossref] [Google Scholar] [PubMed]
- V. Cantarín-Extremera, M. Jiménez-Legido, S. Aguilera-Albesa, A. Hedrera-Fernández, L. Arrabal-Fernández, et al. Opsoclonus-myoclonus syndrome: Clinical characteristics, therapeutic considerations and prognostic factors in a Spanish paediatric cohort, Neurología, 38(2023):93-105.
[Crossref] [Google Scholar] [PubMed]
- A. Mizia-Malarz, W. Stolpa, G. Sobol-Milejska, The treatment of opsoclonus-myoclonus syndrome secondary to neuroblastic tumours-single-centre experience and literature review, Medicina, 56(2020):412.
[Crossref] [Google Scholar] [PubMed]
- P.A. de Alarcon, K.K. Matthay, W.B. London, A. Naranjo, S.C. Tenney, et al. Intravenous immunoglobulin with prednisone and risk-adapted chemotherapy for children with opsoclonus myoclonus ataxia syndrome associated with neuroblastoma (ANBL00P3): A randomised, open-label, phase 3 trial, Lancet Child Adolesc, 2(2018):25-34.
[Crossref] [Google Scholar] [PubMed]
- V.W. Yiu, T. Kovithavongs, L.F. McGonigle, P. Ferreira, Plasmapheresis as an effective treatment for opsoclonus-myoclonus syndrome, Pediatric Neurol, 24(2001):72-74.
[Crossref] [Google Scholar] [PubMed]
- M.B. Armstrong, P.L. Robertson, V.P. Castle, Delayed, recurrent opsoclonus-myoclonus syndrome responding to plasmapheresis, Pediatric Neurol, 33(2005):365-367.
[Crossref] [Google Scholar] [PubMed]
- J.E. Greensher, J. Louie, J.D. Fish, Therapeutic plasma exchange for a case of refractory opsoclonus myoclonus ataxia syndrome, Pediatr Blood Cancer, 65(2018):e26819.
[Crossref] [Google Scholar] [PubMed]
- D.L. Johnston, S. Murray, M.S. Irwin, J. Doyle, T. Schechter, Autologous stem cell transplantation for refractory opsoclonus myoclonus ataxia syndrome, Pediatr Blood Cancer, 65(2018):e27110.
[Crossref] [Google Scholar] [PubMed]
- M.R. Pranzatelli, E.D. Tate, S. Shenoy, A.L. Travelstead, Ofatumumab for a rituximabâ?allergic child with chronicâ?relapsing paraneoplastic opsoclonus–myoclonus, Pediatr Blood Cancer, 58(2012):988-991.
[Crossref] [Google Scholar] [PubMed]
- T.G. Ketterl, Y.H. Messinger, D.R. Niess, E. Gilles, WK. Engel, et al. Ofatumumab for refractory opsoclonusâ?myoclonus syndrome following treatment of neuroblastoma, Pediatr Blood Cancer, 60(2013):163-165.
[Crossref] [Google Scholar] [PubMed]
- K. Ki Pang, B.J. Lynch, J.P. Osborne, M.G. Pike, Dancing eye syndrome associated with spontaneous recovery and normal neurodevelopment, Eur J Paediatr Neurol, 14(2010):178–181.
[Crossref] [Google Scholar] [PubMed]
- A. Galstyan, C. Wilbur, K. Selby, J. Hukin, Opsoclonus-myoclonus syndrome: A new era of improved prognosis?, Pediatr Neurol, 72(2017):65-69.
[Crossref] [Google Scholar] [PubMed]
- E.D. Tate, M.R. Pranzatelli, S.J. Verhulst, S.J. Markwell, D.N. Franz, et al. Active comparator-controlled, rater-blinded study of corticotropin-based immunotherapies for opsoclonus-myoclonus syndrome, J Child Neurol, 27(2012):875-884.
[Crossref] [Google Scholar] [PubMed]
- M.R. Pranzatelli, E.D. Tate, Corrigendum to dexamethasone, intravenous immunoglobulin and rituximab combination immunotherapy for pediatric opsoclonus-myoclonus syndrome, Pediatric Neurol 73(2017)48–56.
[Crossref] [Google Scholar] [PubMed]
- J.B. Abreu, C.R. Cordeiro, A.I. Amorim, T.G. Catanho, K.D, Gama, Combined neuraxial-general anesthesia in opsoclonus–myoclonus syndrome: A case report, Saudi J Anaesth, 17(2023):94-96.
[Crossref] [Google Scholar] [PubMed]
- M. Maranhao, A. Holanda, F. Tavares, Kinsbourne syndrome: Case report, Braz J Anesthesiol, 63(2013):287–289.
[Crossref] [Google Scholar] [PubMed]
- J. Lee, D. Kim, B. Jeon, J.Y. Oh, Y.J. Han, Anesthesia in a young adult with opsoclonus-myoclonus syndrome, Korean J Anesthesiol, 67(2014):5-8.
[Crossref] [Google Scholar] [PubMed]
- M. Kinsbourne, Myoclonic encephalopathy of infants, J Neurol Neurosurg Psychiatry, 25(1962):271.
[Crossref] [Google Scholar] [PubMed]
- N. Nisa, P. Talawar, B. Vasudevan, Anesthesia in a child with Kinsbourne syndrome: Does anesthesia technique matters?, Saudi J Anaesth, 10(2016):468-470.
[Crossref] [Google Scholar] [PubMed]
- S.S. Khatami, M.E. Revheim, P.F. Hoilund-Carlsen, A. Alavi, S.G. Shirkouhi, et al. Central nervous system manifestations following vaccination against COVID-19, Brain Behav Immun Health, 38(2024):100788.
[Crossref] [Google Scholar] [PubMed]
Copyright: © 2025 Lourdes de Fatima Ibanez Valdes, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original work is properly cited.