Case Report: Journal of Drug and Alcohol Research (2025) Volume 14, Issue 4
New Hypotheses on the Pathogenesis of Tolosa-Hunt Syndrome and Drug Therapy
Lourdes de Fatima Ibanez Valdes1 and Humberto Foyaca Sibat2*2Department of Neurology, Nelson Mandela Academic Hospital, Walter Sisulu University, South Africa
Humberto Foyaca Sibat, Department of Neurology, Nelson Mandela Academic Hospital, Walter Sisulu University, South Africa, Email: humbertofoyacasibat@gmail.com
Received: 21-Mar-2025, Manuscript No. JDAR-25-166289; Editor assigned: 25-Mar-2025, Pre QC No. JDAR-25-166289 (PQ); Reviewed: 08-Apr-2025, QC No. JDAR-25-166289; Revised: 05-Aug-2025, Manuscript No. JDAR-25-166289 (R); Published: 12-Aug-2025, DOI: 10.4303/JDAR/236444
Abstract
Introduction: Tolosa‐Hunt Syndrome (THS) is a steroid‐responsive, relapsing‐remitting, unilateral headache disorder associated with ipsilateral cranial neuropathies, due to an Idiopathic Granulomatous Inflammation (IGI), which is a type of chronic inflammation characterized by granuloma formation that contains focal accumulations of immune cells, mainly macrophages, almost always in response to foreign substances, persistent infections or idiopathic conditions. These granulomas can sometimes be associated with sarcoidosis, tuberculosis, other infections or immunerelated disorders. In case of THS the granulomatosis is localized in the Cavernous Sinus (CS) leading to Cavernous Sinus Syndrome (CSS), Orbital Apex (OA) or Superior Orbital Fissure (SOF) causing a superior orbital fissure syndrome
Methods: We searched the medical literature, following the guidelines outlined in the PRISMA statement. From 01st, January 1982 to 31st, January 2025, the authors searched the scientific databases, Scopus, Embassy, Medline, and PubMed Central using the following searches: "Tolosa‐Hunt syndrome" OR "Orbit apex" OR "Superior orbital fissure" OR "Abducens nerve palsy" OR, OR "Cavernous sinus syndrome" OR “Trigeminal nerve” OR “painful ophthalmoplegia”.
Results: After screening the full‐text articles for relevance, n=87 articles were identified through database searching. Records after duplicates removed n=46, Record screened N=33, studies with new proposal of pathogenesis included in final review and meta‐analysis n=0. No article proposing a new hypothesis for pathogenesis of idiopathic granulomatous inflammation responding to drug therapy was found when we searched. Therefore, metanalysis was not performed.
Conclusions: As far we know, this is the first study proposing a new hypothesis for idiopathic granulomatous inflammation responding to steroid therapy.
Keywords
Tolosa-Hunt syndrome; Painful ophthalmoplegia; Cavernous sinus; Diplopia; Corticosteroid therapy; Neuroimaging; Idiopathic granulomatous inflammation
Introduction
Tolosa-Hunt Syndrome (THS) is a steroid-responsive, relapsing-remitting, unilateral headache disorder associated with ipsilateral cranial neuropathies, due to an Idiopathic Granulomatous Inflammation (IGI), which is a type of chronic inflammation characterized by granuloma formation that contains focal accumulations of immune cells, mainly macrophages, almost always in response to foreign substances, persistent infections or idiopathic conditions. These granulomas can sometimes be associated with sarcoidosis, tuberculosis, other infections or immunerelated disorders. In case of THS the granulomatosis is localized in the Cavernous Sinus (CS) leading to Cavernous Sinus Syndrome (CSS), Orbital Apex (OA) or Superior Orbital Fissure (SOF) causing a superior orbital fissure syndrome, also known as Rochon-Duvigneaud syndrome which is a pathological condition caused by granulomatous inflammation affecting structures near the SOF or within it affecting the cranial nerves involved in eye movement, pupil control and sensation, leading to proptosis, ophthalmoplegia, ptosis, and anaesthesia in the forehead and upper eyelid. THS-Idiopathic Granulomatous Inflammation (THS-IGI) differs from Orbital Apex Syndrome (OAS), typically including optic nerve lesion and painful ophthalmoplegia. The exact pathophysiology and aetiology remain unclear, but most investigators believe it to be an immune-mediated inflammatory process that leads to perivascular infiltration, thickening of the cavernous sinus walls, and compression of cranial nerves travelling inside the sinus or through its lateral wall. To assure a good prognosis, early diagnosis is mandatory, as prompt drug therapy with corticosteroids results in rapid resolution of all symptoms and signs. THS-IGI remains a challenging diagnosis for many physicians, paediatricians and neurologists [1-6].
In 1954, Tolosa first described this syndrome, which was later expanded by Hunt et al., in 1961. Dr. Eduardo Tolosa, a Spanish neurosurgeon, and Dr. William Hunt independently reported patients with ophthalmoplegia with evidence of granulomatous inflammation in the cavernous sinus.
Subsequently, this eponym was applied to patients with "investigation negative" Cavernous Sinus Syndrome (CSS) [6,7].
Seven years after his first publication, Hunt reported a series of six cases and established the parameters to diagnose this condition, as follows:
• Alterations on the third cranial nerve, throclear nerve, abducens nerve, or first branch of the fifth cranial trigeminal nerve and, less commonly, involvement of the optic nerve or sympathetic fibres around the cavernous portion of the carotid.
• Acute retro-orbital pain.
• Symptoms persisting for days or weeks.
• Spontaneous pain remission.
• Recurrent episodes.
• Prompt response to steroids [8].
In most cases, presenting THS-IGI typically complain of acute or subacute onset of unilateral periorbital pain, followed by oculomotor (CN III), trochlear (CN IV), and abducens (CN VI) nerve involvement leading to diplopia, ptosis, and ophthalmoplegia. The pain is often quite intense and persistent, poorly responding to conventional analgesics. Weight loss and fever are usually absent, which serves to clinically differentiate THS from malignant disorders or infectious diseases [9,10]. MRI is essential for confirming the final diagnosis. In many patients, it shows enlargement and enhancement of the CS extending into the OA and SOF [11].
Cerebrospinal Fluid (CSF) studies and laboratory investigations are usually normal, which supports the differential diagnosis with other conditions such as malignancies, infections, and systemic vasculitis such as granulomatosis with polyangiitis [12].
Recently, Park et al. reported the first case presenting a Tolosa-Hunt syndrome with AQP4-immunoglobulin G seropositivity [13].
This study intends to highlight the main features of the clinical manifestations, differential diagnosis, and drug therapy of Tolosa-Hunt syndrome, emphasizing the role of MRI images in confirming the final diagnosis and the efficacy of corticosteroid therapy.
Many years after the first description of this condition, the pathophysiological mechanisms of the disease remain obscure and controversial. Therefore, the main aim of this study is to find the necessary information to respond to the following research question: What is the most likely pathophysiology of THS?
Materials and Methods
Search strategy
We searched the medical literature, following the guidelines outlined in the PRISMA statement. From 01st, January 1982 to 31st, January 2025, the authors searched the scientific databases, Scopus, Embassy, Medline, and PubMed Central using the following searches: "Tolosa-Hunt syndrome" OR "Orbit apex" OR "Superior orbital fissure" OR "Abducens nerve palsy" OR, OR "Cavernous sinus syndrome" OR “Trigeminal nerve” OR “painful ophthalmoplegia.” After removing duplicates, two reviewers (LDFIV and HFS) indenpendently screened titles and abstracts and evaluated the full texts of eligible articles based on the proposed inclusion criteria. Any disagreement between the reviewers involved in the literature search was resolved through discussion with all authors to reach a consensus.
Selection criteria
The following manuscripts were included in the systematic review and meta-analysis: Articles on with detailed pathogenesis and/or drug therapy.
Exclusion criteria were as follows:
• Inaccessibility to full text.
• Articles with unclear pathogenesis.
• Lack of relevant clinicopathological data.
• Non-original studies (i.e., editorials, letters, conference proceeding, book chapters).
• Animal model studies.
• Non-/Spanish/Portuguese/English studies.
The papers were thoroughly assessed, and duplicates were looked for.
Data extraction and quality assessment
All selected data were tabulated in an electronic Excel database. That information included pathogenesis, drug management, initial clinical presentation, evaluation of after treatment, follow-up, and status at the latest evaluation. The studies' quality was categorized as good, poor, fair, or reasonable, in agreement with the National Institutes of Health criteria.
Statistical analysis
The primary objective of this study was to evaluate whether reported pathogenesis/drug management differs significantly among different therapies. Without a comprehensive reference for the total number of cases, the prevalence of responding to drug therapy with associated autonomic manifestations was searched through a comprehensive review.
Statistical analyses were performed using XLSTAT (add-on for Microsoft Excel, version 2021.4.1, Addinsoft SARL) and RStudio (version 4.3.1, https://www.rstudio.com/). Variations in continuous variables were assessed using the Mann-Whitney U-test. We presented descriptive statistics for continuous variables as median (95% Confidence Interval (95% CI)). All situations were evaluated using the Kaplan-Meier method to identify relevant prognosticators. A model of multivariable Cox proportional hazards with a priori selection of covariates was used to check for independent prognostic effects.
Results and Discussion
A total of 87 titles were selected from the literature. After removing duplicates and excluding records 46 relevant articles were examined. Thirteen studies were unavailable for retrieval. After including six additional articles identified from citation searching, 33 were excluded for several reasons. A total of eight records were identified from these searches. However, no publications regarding new hypotheses on pathogenesis of idiopathic granulomatous inflammation responding to steroid therapy was found. We screened all remaining unique full-text articles, abstracts, and titles for eligibility. Titles were typically excluded if unavailable in Spanish, Portuguese, or English or irrelevant to THS. Articles relevant to THS included the clinical presentation, management, and treatment. No articles were for full-text review for meta-analysis because they no one delivered a new hypothesis on THS-IGI.
Series description and differences among groups
All the selected studies were relevant to the subject of this systematic review. None were randomized controlled trials or prospective studies; all the articles included were case reports and case series. The total number of patients presenting with documented xxx was zero.
Median age was 14.7 (range 5-71) with significant differences between age groups (p<0.001). We did not find remarkable variations in gender (p=0.064), although males presenting GS were noticeably more frequent and slightly more prevalent.
Comments and final remarks
The hallmark of THS-IGI is the awe-inspiring response to the drug therapy with corticosteroids showing a spectacular rapid resolution of symptoms and signs within 24 to 72 hours after the treatment began, which can be accepted as a key diagnostic criterion for confirmation.
Notwithstanding, some relapses are commonly expected to be seen. Therefore, long-term follow-up is necessary to identify steroid dependency and recurrence. Although in refractory cases, methotrexate or azathioprine as an alternative immunosuppressive agent should be prescribed, as has been proposed by other authors many years ago [14].
Based on THS-IGI rarity and diagnostic complexity, this syndrome commonly poses a clinical challenge. To reach a high degree of clinical suspicion, early diagnosis and proper management are necessary because they are crucial in preventing unnecessary interventions and ensuring good patient outcomes.
The International Classification of Headache Disorders (ICHD) classifies THS-IGI as a distinct entity characterized by cranial nerve palsies, severe periorbital pain, and rapid corticosteroid response [1,15].
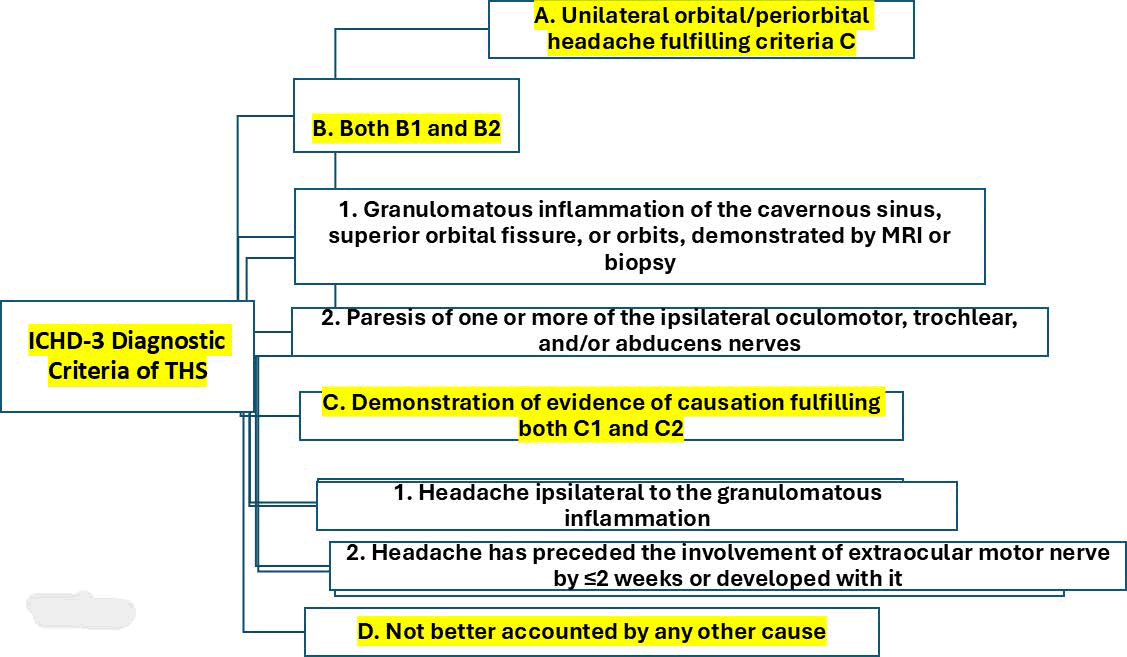
In 2013, the diagnostic criteria for Tolosa-Hunt syndrome were updated in the 3rd Edition of the International Classification of Headache Disorders; since then, it is possible to diagnose this syndrome based on the presence of granulomatous inflammation confirmed on magnetic resonance imaging without confirmation by biopsy [16]. The ICHD-3 diagnostic criteria of THS are summarised in Figure 1.
Figure 1: Showing the diagnostic criteria of THS-IGI
As we will comment below, we defined nonspecific granulomatous inflammation as a chronic inflammation characterized by granuloma formation, including a collection of immune cells, in response to various stimuli rather than a specific cause.
Here, we highlight that THS-IGI is a diagnosis of exclusion. A long list of medical and surgical causes of painful ophthalmoplegia must be differentiated from THS-IGI as a differential diagnosis. Table 1 lists all associations of other autoimmune disorders reported in the medical literature (Table 1).
| Causes | Differential diagnosis |
| Trauma | |
| Vascularcauses: | Posterior communicating artery aneurysm Cavernous ICA aneurysm Carotid-cavernous fistula/dissection Cavernous sinus thrombosis |
| Diabetes: | Diabetic ophthalmoparesis |
| Infiltrative: | Superior orbital fissure, the orbit's apex, THS-GI |
| Infectious: | Bacterial sinusitis, periostitis, orbital cellulitis, syphilis. Viral: Herpes zoster Fungal: Mucormycosis Mycobacterium tuberculosis |
| Non-infectious: | Polyneuritis cranialis, sarcoidosis, IgG4-related disorder, eosinophilic granuloma, vasculitis Wegener's granulomatosis, giant-cell arteritis, orbital inflammatory syndrome, hypertrophic pachymeningitis, Recurrent Painful Ophthalmoplegic Neuropathy, lymphoma, SLE and PO. |
| Vascular headache: | Ophthalmoplegic migraine |
| Neoplastic lesions: | Nasopharyngeal carcinoma, melanoma, Burkitt |
Table 1: Main causes of painful ophthalmoplegias.
Table 1 showed the conditions and differential diagnoses that should be adequately ruled out before diagnosing THS-IGI [17].
One of the medical conditions included in Table 1 that should be distinguished from THS-IGI is the IgG4-related ophthalmic spectrum of disorders, which is usually seen in three distinctive types:
• Enlargement of the infra-orbital nerve.
• Compressive optic neuropathy.
• Extraocular myositis.
This differentiation based on the high serum levels of IgG4 should be done to ensure that no pathological condition mimicking IgG4 spectrum disorder through raised IgG4 levels gets mistakenly labelled while missing the primary diagnosis. Recently, THS-IGI has been reported as an initial manifestation of systemic lupus erythematosus [18].
Brief comments on epidemiology
THS is a rare medical pathology with an incidence of around 1-2 cases per million per year. It constitutes nearly 23% of the causes of CSS. To date, no racial or geographical predominance has been confirmed. The main age of onset has been identified to be in the fourth decade, and THS does not show predominance for any gender [17].
The clinical features of THS can be simultaneous, unilateral, sequential, or bilateral. Pain is often around the eyeball or brow on the affected side or can be retroorbital or even extend to the frontal or temporal regions. It has been suggested that a localized dural granulomatous inflammation precedes ophthalmoplegia with a duration of up to fourteen days, and on average lasts for about two months in untreated cases. All extraocular muscles can be affected in any combination. Proptosis may happen in a few patients due to the involvement of peri-arterial sympathetic fibres leading to contraction of Muller's muscle. The third nerve is the most affected in about 80% of the cases, followed by the abducens nerve, which is involved in 70% of patients. In 20% of patients, a thirdorder Horner's syndrome secondary to involvement of the Oculo-sympathetic plexus around the internal carotid artery can be observed, and the trochlear nerve and ophthalmic and maxillary divisions of the fifth cranial nerve can be affected in several combinations. Although less common, the spread of granulomatous inflammation anteriorly into the superior orbital fissure or the orbital apex may cause damage to the optic nerve, leading to blindness, papillitis and consequent optic pallor. An extension can involve the vestibulocochlear nerve, the facial nerve, and other vital structures if an extension occurs posteriorly. Other clinical manifestations, such as vomiting, nausea, and fatigue secondary to severe periorbital pain, have been published in anecdotal case reports/series [6,19].
Brief comments on the role of imagenology for THS-IGI
Usually, the conventional neuroimaging techniques do not show the proper visualization of the CS. Therefore, memorable fat-saturated, contrast-enhanced, Turbospin Echo T1 and T2 sequences in the axial and coronal sections must be performed in the study of the CS. The superior orbital fissure, orbital apex, and anterior temporal lobe should be carefully examined in THS patients. Undoubtedly, a more remarkable contrast-enhanced MRI is the investigation of choice for diagnosing THS. The most important findings include enhancement and enlargement of the SOF, CS, or OA, usually with asymmetric thickening of the dura mater [20]. The characteristic description of THS is convexity of the lateral wall of the CS due to the presence of soft tissue, which appears isointense on T1, hypointense on T2, enhances upon contrast administration, and is diffusionweighted imaging restricted. On the other hand, a bulging contour of the lateral wall of the CS, dural meningeal enhancement (focal pachymeningitis) of neighbouring structures, and focal narrowing of the intracavernous ICA are other subtle signs suggesting THS [6]
High-resolution CT scans can demonstrate bony changes such as erosion, hyperostosis, calcification, soft tissue inflammation, and associated haemorrhage. However, they have limited sensitivity for CS/OA lesions and limitations such as bony artefacts and superimposed beam hardening [21].
Cerebral angiography is important to rule out fistulae in the carotid–cavernous territory, and some radiological findings such as irregularity, local narrowing, or constriction of the intracavernous carotid artery can help in the differential diagnosis of THS-granulomatous. Some authors have described soft tissue inflammation as causing intense constriction of the carotid siphon, leading to high resistance flow, which is described as the "arterial stationary wave phenomenon" in older publications on THS [22].
Imai et al., reported a patient with THS-IGI in whom arterial spin labelling was used to diagnose the condition and monitor the therapeutic response following steroid therapy. This technique, performed routinely and repeatedly without ionizing radiation or contrast administration, can help diagnose THS-IGI [23].
Despite some limitations because of its low specificity, Fluorodeoxyglucose (FDG) positron emission tomography may provide valuable information to differentiate THS-IGI from other ominous disorders due to its capacity to show focal hypermetabolism, suggesting inflammation in THS [24].
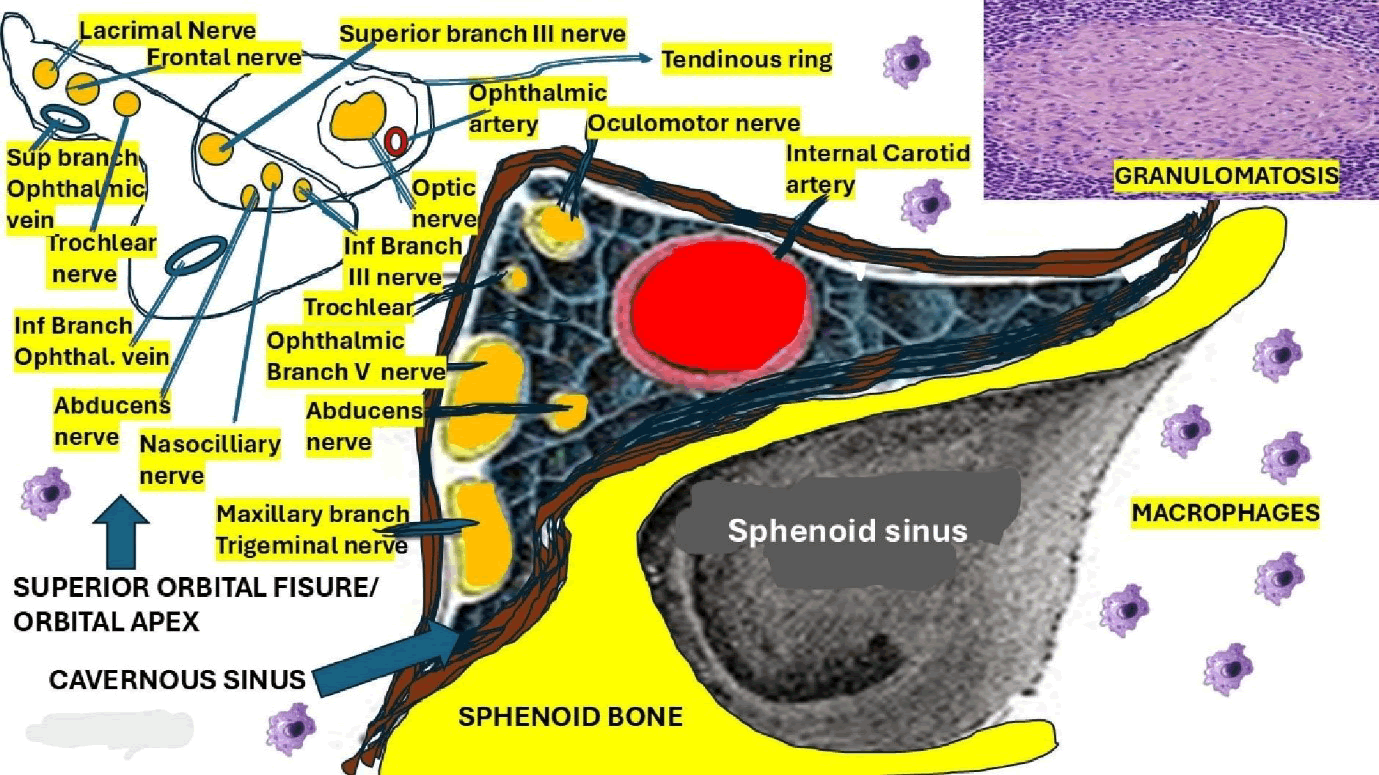
Local biopsy is the best procedure to confirm granulomatous inflammation, but it is unnecessary and rarely performed because of the good response to drug therapy and its invasiveness [25]. Therefore, obtaining a biopsy sample can be very hard due to the deep location of the CS and the large number of cranial nerves around it, which can be unnecessary (Figure 2). The presence of abundant lymphocytes/plasma cells, epithelioid, fibroblasts, and occasional giant cells infiltrating the septa and the walls of cavernous sinuses will confirm the diagnosis of THS-IGI.
Figure 2: Shows the gross anatomy of the CS, SOF, OA and all nerves included with the presence of macrophages as part of the idiopathic granulomatous inflammatory process
Brief comments on drug therapy
The hallmark treatment for THS-IGI is high-dose corticosteroids, which lead to the rapid resolution of symptoms and signs in almost all patients. The corticosteroids dramatically suppress the granulomatous inflammation within the CS and around the OA and SOF [26], one of the supporting criteria for confirmation of THS-IGI. Despite this, relapse can be observed in up to 30%-40% of patients, requiring close follow-up and longterm immunosuppressive therapy [27].
High-dose pulse steroid therapy has been recommended in the medical literature, and we suggest starting with a 3-day course of 1000 mg of intravenous methylprednisolone followed by oral prednisone (0.75 mg/kg) in tapering doses for a duration of one and a half to two months. There is no universal agreement on the optimal dose, duration of therapy, route of administration, and treatment protocols for a special subset of patients (pregnant patients, children, etc.). Proper improvement of symptoms and signs like periorbital/orbital pain usually starts within 24 hours, while cranial nerve recovery takes 6 to 8 weeks [6,28].
If the patient does not respond to the steroid therapy, the diagnosis of THS-IGI should be questioned. The regular restoration of the imagenology findings will take place after the clinical improvement by weeks or even several months [29]. However, some patients may have residual cranial nerve palsies [6]. Some authors report that relapses may occur even months after the first episode in around 39% of patients [6], in which situations long-term steroid therapy is recommended. Notwithstanding, the role of steroid-sparing agents in preventing relapse has not been well documented yet. However, in cases presenting relapse after administration of steroids, the use of other immunosuppressive/immunomodulatory drugs such as methotrexate, azathioprine, mycophenolate mofetil, rituximab, tacrolimus, infliximab, cyclophosphamide, cyclosporine, Tumour Necrosis Factor-a (TNF-a) antibody, and adalimumab should be considered as supplementary therapy [6].
In patients who are partially responsive, non-responsive, or who are non-tolerant to medical treatment or in whom classical medical therapy is contraindicated, focal radiotherapy/gamma knife radiosurgery can be implemented [30].
The overall outcome of THS-IGI is good, even though up to 40% of cases can relapse after complete improvement [31,32].
Cases presenting with Painful Ophthalmoplegia (PO), even if they meet THS-IGI diagnostic criteria, must all be followed for at least two years for clinical progress and recurrence [33,34].
Our hypotheses on the pathogenesis of THS-idiopathic granulomatous inflammation
Based on our previous hypotheses related to the pathogenesis of granuloma formation in neurosarcoidosis [35], we propose a quite similar mechanism for the pathogenesis of THS-IGI. In Figure 2, we represented all elements involved in the pathogenesis of THS-IGI, including the anatomical locations of all cranial nerves affected by the granulomatous inflammation, with the participation of macrophages at the CS, OA and SOF seen in this syndrome.
We believe that some environmental features, microbes, and occupational activities should be considered in their pathological process, including some genetic factors, inhalations of antigens, and the hyperproduction of Th1 cytokines like TNF-α, IFN-γ, and IL-1, among others. Other authors have documented the participation of this inflammatory-mediated response mechanism in the production of granulomas [36-42].
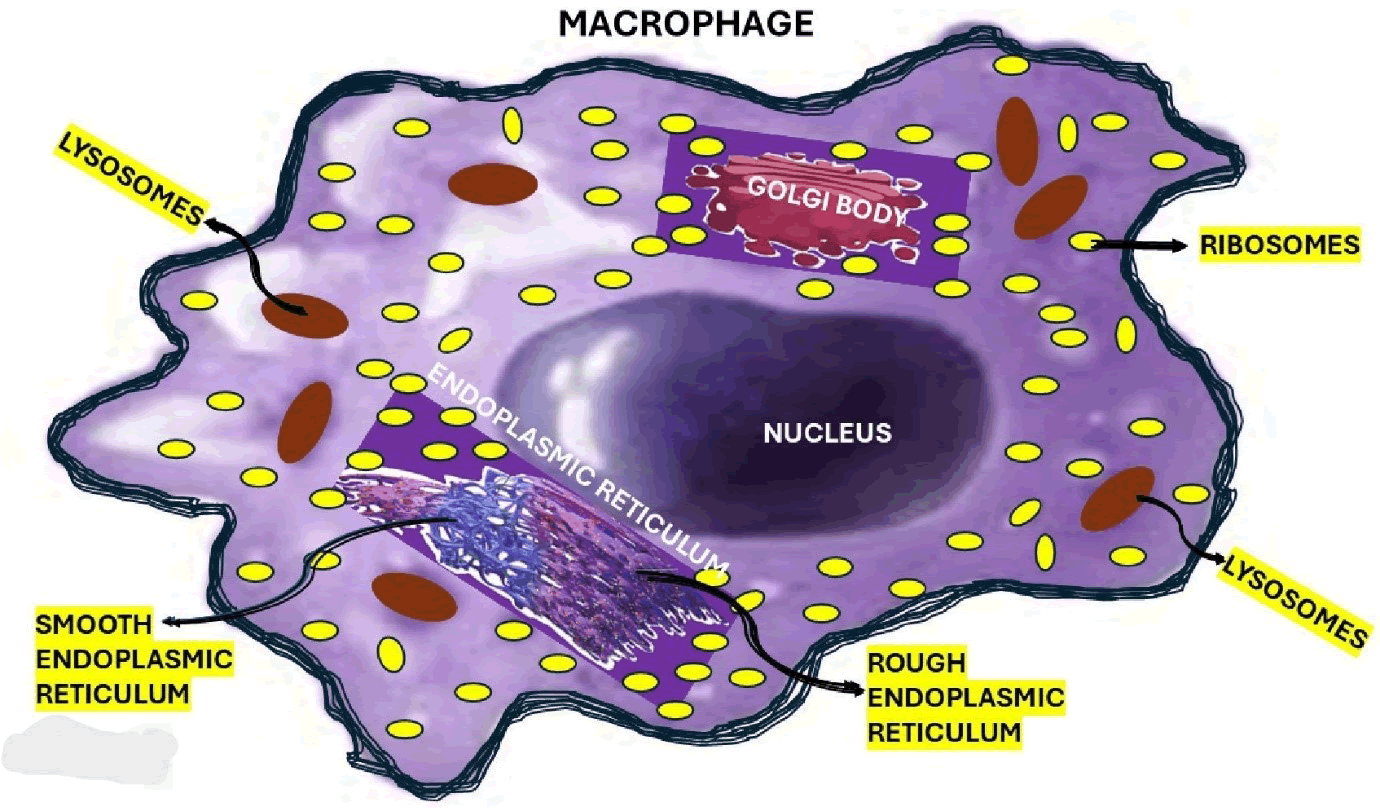
In answering our research question, we hypothesized that macrophages are the most involved element in the pathogenesis of THS-IGI. In Figure 3, we represented the histological components of the macrophages, and below, we will comment on their role (Figure 3).
Figure 3: Show all elements of the macrophages including the graphical representation of the endoplasmic reticulum and Golgi apparatus.
Notwithstanding, we hypothesized that most types of THS-IGI are a direct consequence of a Hyperinflammatory State (HIS) with increased TNF alpha, C-reactive protein, IFN-1-beta, and ferritin. Other investigators documented the relationship between HIS and subsequent activation of astrocytes and microglia injuring the CNS, optic nerves, and SC [43-45].
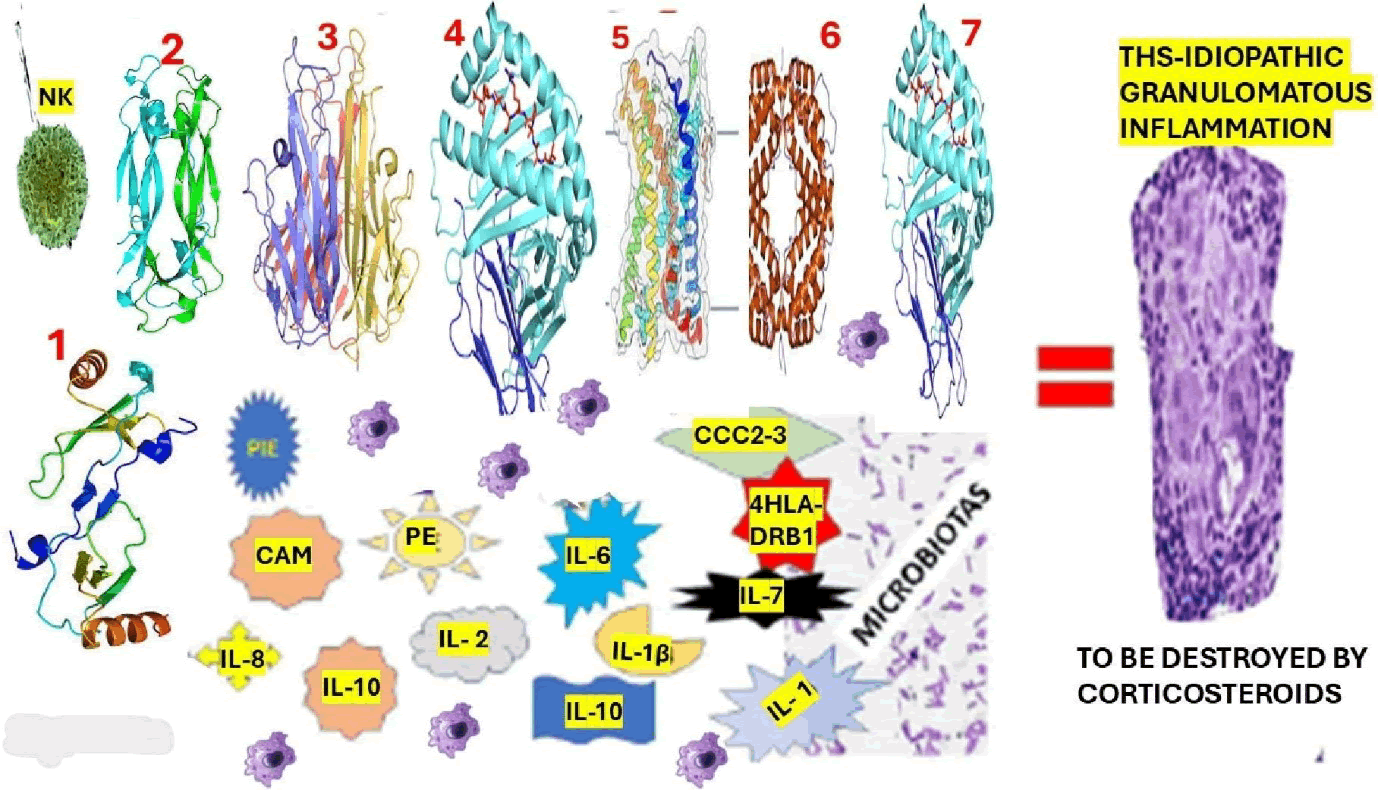
Based on the previously mentioned reports and the studies made by our group related to granulomatous inflammation in other granulomatous (sarcoidosis), we hypothesized the elements involved in the pathogenesis of THS-IGI (Figure 4).
Figure 4: Graphical representation of our hypothesis on the pathogenesis of THS-Idiopathic granulomatous inflammation. Note: The proposed elements participating in this process are as follows: 1. CCL2: Also named Monocyte Chemoattractant Protein 1 (MCP-1), is a cytokine which plays a vital role in immune responses. 2. IL-17: Interleukin-17 is a type of protein (cytokine) that plays a crucial role in all immune system activities, mainly in host defence and inflammation, and plays a remarkable role in granuloma formation. 3. TNFα: Tumour Necrosis Factor-alpha is a vital cytokine (signalling protein), primarily produced by immune cells to regulate the immune response. 4. HLADRB1: Is a gene that plays a vital role in the immune system. It is part of the HLA complex, leading the immune system to differentiate between self and non- self-proteins. 5. CCR 5, is a chemokine receptor interacting with HIV-1 envelope, in association with CD4 and may be a marker for favourable prognosis for renal cancer, Ewing's sarcoma, and colon cancer., 6 IFN-γ: Interferon-gamma is a cytokine (signaling molecule) produced by T cells and Natural Killer (NK) cells and plays a crucial role in modulating several immune and inflammatory processes. 7. CCC2- 3: Chemokine C-C motif ligands 2 and 3 are important chemokines in immune cell migration and inflammation. While CCL2 is a remarkable chemoattractant for monocytes, both CCL2 and CCL3 participate in T cell recruitment. NK: Natural killer cells are leukocytes belonging to the innate immune system, which are relevant for host defence and immune surveillance against cancerous cells and viruses and can kill these cells without prior sensitization or activation, unlike other immune cells like cytotoxic T cells. PIE: Proinflammatory Enzyme is released from neutrophils, which plays a vital role in inflammatory processes. Some PIEs, like MPO, COX-2, iNOS, and MMP, promote inflammation and can contribute to tissue damage.
Arguably, the presence of the HLA-DRB1*03 allele in cases presenting with Lofgren syndrome (human leukocyte antigen) [35] also supports our hypothesis.
Based on our previous investigations, we hypothesized that both environmental antigens (infectious and noninfectious) may facilitate the development of THS-IGI [35]. In all cases, macrophages play a remarkably high role.
Figure 4 represents the mechanism of THS-IGI, which may clarify the neurological manifestations of THS-IGI secondary to the pathological disruption of the involved cranial nerve tissue microarchitecture caused by the granuloma's compression, as has been reported in cases presenting blindness and hypopituitarism secondary to hypothalamic-pituitary sarcoidotic granulomas [46].
Based on our previous study, we also hypothesized that THS-IGI (Figure 4) are composed of fibroblasts with scattered B cells, activated microglia, T helper cells within a ring of CD8+ cytotoxic, other proinflammatory cytokines, and astrocytes supported by TNF-α, IFN-β/γ, IL-2, and CCC2-3: Chemokine C-C motif ligands 2 and 3, among others which, through stimulating naive CD4+T cell produce and maintenance of THS-IGI. In addition, it is essential to remember that IFN-β and IL-2 are produced by T helper cells, increasing the proliferation of immune cells plus cytotoxicity, and Th17- polarised effector T cells have been found in THS-IGI, which can modulate the THS outcome.
We have hypothesized that this hyperinflammatory response is caused by TNF-α, IFN-β/γ, IL-2, and CCC2-3, HLADRB1, CCR 5a, PIE, NK, among others, cause disruption of the average balance between Firmicutes and Bacteroidetes in the intestine causing dysbiosis, neurotransmitter disturbances, and neurohormonal dysfunction from the enteric nervous system causing additional damage on the brain via gut-microbiota-brain axis which may contribute to cavernous sinus, superior orbital fissure and orbital apex granulomatosis as its summarized in Figure 3.
Conclusion
Summarizing, THS-IGI is characterized by steroidresponsive painful ophthalmoplegia from idiopathic GI of the CS, SOF, or OA, which falls under the Idiopathic Orbital Inflammatory (IOI) diseases, which also includes orbital pseudotumor, visual impairment, distinguishing lesions of the OA (optic nerve involvement) from the CSS. THS-IGI is a diagnosis of exclusion, and an extensive workup is necessary for the broad differential diagnosis of PO. Steroid-responsiveness is not specific to THS-GI, and sometimes patients need long-term monitoring and imaging to ensure remission. Intracranial extension is uncommon but may occur in THS-IGI and IOI.
As far we know, this study is the first proposing a new hypothesis for idiopathic granulomatous inflammation in THS responding to steroid therapy.
Author Contributions
Both investigators have read and agreed to the published version of the manuscript.
Conflict of Interest Statement
The authors declare they have no conflicts of interest.
Funding Information
The authors received no funds to perform the present research.
Ethics Statement
The study was conducted using the principles of the Helsinki Declaration, the Italian and US privacy and sensitive data laws, and the internal regulations for retrospective studies of the Otolaryngology Section at Padova University and Brescia University.
Data Availability Statement
The corresponding author will make the raw data supporting this article's conclusions available upon request.
Acknowledgments
The authors thank Prof Thozama Dubula, Head of Department on Internal Medicine and Therapeutic for his unconditional support in the management of this patient.
References
- R. Yadav, P. Parihar, P. Bhangale, U. Jajoo, Tolosa-Hunt syndrome presenting as persistent unilateral headache and painful ophthalmoplegia: A rare case report highlighting diagnostic challenges and therapeutic response, Radiol Case Rep, 20(2025):3340-3343.
[Crossref] [Google Scholar] [PubMed]
- N. Sim, S. Liew, D.J. Warren, A. Hassan, TolosaâHunt syndrome presenting with features of a trigeminal autonomic cephalalgias and pituitary enlargement, BMJ Case Rep, 15(2022):e246519.
[Crossref] [Google Scholar] [PubMed]
- S. Neupane, P. Pudasaini, B. Dhakal, P. Sherpa, P. Rokaya, et al. Recurrent TolosaâHunt syndrome: A case report, Ann Med Surg (Lond), 86(2024):1695–1699.
[Crossref] [Google Scholar] [PubMed]
- M. Mohebbi, S. Nafissi, M. Alikhani, A New Case of Granulomatosis with Polyangiitis Presented with Tolosa-Hunt Syndrome Manifestations. Case Rep Rheumatol. 2024(2024):5552402.
[Crossref] [Google Scholar] [PubMed]
- K. Morooka, S. Kawamoto, M. Shimozawa, R. Tateishi, F. Haba, et al. Thoracic endovascular aortic repair for descending thoracic aortic thrombus of a patient with TolosaâHunt syndrome, J Surg Case Rep, 2025(2025):208.
[Crossref] [Google Scholar] [PubMed]
- A.T. Kapila, S. Ray, V. Lal, Tolosa–Hunt Syndrome and IgG4 Diseases in Neuroâ Ophthalmology, Ann Indian Acad Neurol, 25(2022):S83–S90.
[Crossref] [Google Scholar] [PubMed]
- P. Dutta, K. Anand, Tolosa–Hunt syndrome: A review of diagnostic criteria and unresolved issues, J Curr Ophthalmol, 2021;33:104–111.
[Crossref] [Google Scholar] [PubMed]
- Hunt WE, Tolosa-Hunt syndrome: One cause of painful ophthalmoplegia, J Neurosurg, 1976;44(5):544–549.
[Crossref] [Google Scholar] [PubMed]
- R. Shree, K.V. Mahesh, N. Balaini, A. Goel, Oculomotor cranial neuropathies: Diagnosis and management, Ann Indian Acad Neurol, 2022;25:S70–S82.
[Crossref] [Google Scholar] [PubMed]
- N.K. Sim, S.Y. Liew, D.J. Warren, A. Hassan, TolosaâHunt syndrome presenting with features of a trigeminal autonomic cephalalgias and pituitary enlargement, BMJ Case Rep, 15(2022):e246519.
[Crossref] [Google Scholar] [PubMed]
- P. Goyal P, S. Lee S, N. Gupta, Orbital apex disorders: Imaging findings and management, Neuroradiol J, 31(2018):104–125.
[Crossref] [Google Scholar] [PubMed]
- P. Berlit, M. Kraemer, Cerebral vasculitis in adults: What are the steps in order to establish the diagnosis? Red flags and pitfalls, Clin Exp Immunol, 175(2014):419–424.
[Crossref] [Google Scholar] [PubMed]
- S.H. Park, S.I. Jang, E.J. Lee, N.H. Kim, Case report: TolosaâHunt syndrome-expanding the neuromyelitis optica spectrum disorder phenotype?, Front Neurol, 15(2024):1326867.
[Crossref] [Google Scholar] [PubMed]
- A. Furuse, M. Hiramatsu, N. Adachi, S. Karashima, S. Hattori, et al. Dramatic response to corticosteroid therapy of nephrotic syndrome associated with IgA nephropathy, Int J Pediatr Nephrol, 6(1985):205-208.
[Google Scholar] [PubMed]
- P.R. ChÄ
dzynski, K. Stopinska, I. Domitrz, TolosaâHunt syndrome: A review of diagnostic criteria based on a case series, Postep Psychiatr Neurol, 33(2024):26-34.
[Crossref] [Google Scholar] [PubMed]
- M. Imai, A. Sunaga, R. Aoki, T. Osada, K. Hoshikawa, et al. Possibility of arterial spin labeling perfusion magnetic resonance imaging sequences with steroid therapy for TolosaâHunt syndrome: A case report and review of literature, Surg Neurol Int, 13(2022):27.
[Crossref] [Google Scholar] [PubMed]
- L. Kline, W. Hoyt, The Tolosa-Hunt syndrome, J Neurol Neurosurg Psychiatry, 71(2001):577–582.
[Crossref] [Google Scholar] [PubMed]
- F. Nilofar, K. Mohanasundaram, M. Kumar, T. Gnanadeepan, TolosaâHunt Syndrome as the Initial Presentation of Systemic Lupus Erythematosus, Cureus, 16(2024):e61692.
[Crossref] [Google Scholar] [PubMed]
- Kline LB, The TolosaâHunt syndrome, Surv Ophthalmol, 27(1982):79-95.
[Crossref] [Google Scholar] [PubMed]
- S.R. Vallejo, R.A. Lopez, A.M. Olarte, S.L. Roman, MRI findings in Tolosa-Hunt syndrome (THS), BMJ Case Rep, 2014(2014).
[Crossref] [Google Scholar] [PubMed]
- S. Bhatkar, K.V. Mahesh, J. Sachdeva, A. Goel, M.K. Goyal, et al. Magnetic Resonance Imaging (MRI) versus Computed Tomographic scan (CT scan) of brain in evaluation of suspected cavernous sinus syndrome, Neuroradiol J, 33(2020):501–507.
[Crossref] [Google Scholar] [PubMed]
- H.L. Kettler, J.D. Martin, Arterial stationary wave phenomenon in TolosaâHunt syndrome, Neurology, 25(1975):765–770.
[Crossref] [Google Scholar] [PubMed]
- M. Imai, A. Sunaga, R. Aoki, T. Osada, K. Hoshikawa, et al. Possibility of arterial spin labeling perfusion magnetic resonance imaging sequences with steroid therapy for TolosaâHunt syndrome: A case report and review of literature, Surg Neurol Int, 13(2022):27.
[Crossref] [Google Scholar] [PubMed]
- Y.C. Wu, T.C. Hsieh, C.H. Kao, Y.L. Liu, K.Y. Yen, et al. A rare case of Tolosa-Hunt syndrome imaged with FDG PET/CT and MRI, Clin Nucl Med, 36(2011):574–575.
[Crossref] [Google Scholar] [PubMed]
- V. Koo, T. Lioe, R. Spence, Fine Needle Aspiration Cytology (FNAC) in the diagnosis of granulomatous lymphadenitis, Ulster Med J, 75(2006):59–64.
[Google Scholar] [PubMed]
- P.R. Nobrega, P.G.B. Rodrigues, I.S. Pereira, Steroid responsive cavernous sinus syndrome due to RosaiâDorfman disease: Beyond TolosaâHunt syndrome-a case report, BMC Neurol, 21(2021):264.
[Crossref] [Google Scholar] [PubMed]
- K. Bennett, E. Boccio, One in a Million: A Woman Presenting with Unilateral Painful Ophthalmoplegia, Clin Pract Cases Emerg Med, 8(2024):176–178.
[Crossref] [Google Scholar] [PubMed]
- Y. Sabrina, T. Chen, Pearls & Oyâsters: Idiopathic Orbital Inflammation and TolosaâHunt Syndrome with Intracranial Extension, Neurology, 101(2023):371–374.
[Crossref] [Google Scholar] [PubMed]
- S. Cakirer, MRI findings in Tolosa-Hunt syndrome before and after systemic corticosteroid therapy, Eur J Radiol, 45(2003):83–90.
[Crossref] [Google Scholar] [PubMed]
- E. Mormont, P. Laloux, J. Vauthier, M. Ossemann, Radiotherapy in a case of TolosaâHunt syndrome, Cephalalgia Int J Headache, 20(2000):931–933.
[Crossref] [Google Scholar] [PubMed]
- A. Arthur, A. Sivadasan, P. Mannam, A.T. Prabakhar, S. Aaron, et al. Tolosa-Hunt syndrome: Long-term outcome and role of steroid-sparing agents, Ann Indian Acad Neurol, 23(2020):201–205.
[Crossref] [Google Scholar] [PubMed]
- B.S. Beraldin, A. Felippu, F. Martinelli, H.C. Patricio, TolosaâHunt syndrome mimicking cavernous sinus tumor, Braz J Otorhinolaryngol, 79(2015):256.
[Crossref] [Google Scholar] [PubMed]
- E. Ertilav, A. Akyol, Evaluation of Patients with Painful Ophthalmoplegia for Benign and Secondary Etiologies, Neuroophthalmology, 48(2024):338–347.
[Crossref] [Google Scholar] [PubMed]
- S. Bowman, A. Helming, Complete ophthalmoplegia diagnosed as TolosaâHunt syndrome on interval MRI, BMJ Case Rep, 15(2022):e252727.
[Crossref] [Google Scholar] [PubMed]
- F.S. Humberto, Unique Presentation of Neurosarcoidosis: Case Report and Systematic Review, Clin Schizophr Relat Psychoses, 16(2022).
- S. Weigt, S. Samuel, V. Palchevskiy, J.A. Belperio, Inflammasomes and ILâ1 Biology in the Pathogenesis of Allograft Dysfunction, J Clin Invest, 127(2017):2022â2029.
[Crossref] [Google Scholar] [PubMed]
- Kron, Jordana, A.G. Mauro, A. Bonaventura, S. Toldo, et al. Inflammasome Formation in Granulomas in Cardiac Sarcoidosis, Circ Arrhythm Electrophysiol, 12(2019):e007582.
[Crossref] [Google Scholar] [PubMed]
- Huppertz, Christine, B. Jager, G. Wieczorek, P. Engelhard, et al. The NLRP3 inflammasome pathway is activated in sarcoidosis and involved in granuloma formation, Eur Respir J, 55(2020).
[Crossref] [Google Scholar] [PubMed]
- Moller, R. David, B.A. Rybicki, N.Y. Hamzeh, C.G. Montgomery, et al. Genetic, Immunologic, and Environmental Basis of Sarcoidosis, Ann Am Thorac Soc, 14(2017):S429âS436.
[Crossref] [Google Scholar] [PubMed]
- V. Moorsel, H.M. Coline, C. David, Genetic Susceptibility to Sarcoidosis, a Chronic Inflammatory Disorder, Am J Respir Crit Care Med, 186(2012):816â818.
[Crossref] [Google Scholar] [PubMed]
- Sibat, H. Foyaca, Comorbidity of Neurocysticercosis, HIV, Cerebellar Atrophy and SARSâCoVâ2: Case Report and Systematic Review, Clin Schizophr Relat Psychoses, 15(2022):1â6.
- Sibat, H. Foyaca, Neurocysticercosis, Epilepsy, COVIDâ19 and a Novel Hypothesis: Cases Series and Systematic Review, Clin Schizophr Relat Psychoses, 15(2021):1â13.
- Grunewald, Johan, A. Eklund, Lofgren's Syndrome: Human Leukocyte Antigen Strongly Influences the Disease Course, Am J Respir Crit Care Med, 179(2009):307â312.
[Crossref] [Google Scholar] [PubMed]
- Rose, R. Noel, C. Bona, Defining Criteria for Autoimmune Diseases (Witebsky's Postulates Revisited), Immunol Today, 14(1993):426â430.
[Crossref] [Google Scholar] [PubMed]
- Fingerlin, E. Tasha, N. Hamzeh, L.A. Maier, Genetics of Sarcoidosis, Clin Chest Med, 36(2015):569â584.
[Crossref] [Google Scholar] [PubMed]
- Anthony, Jeremy, G.J. Esper, A. Ioachimescu, Hypothalamic-Pituitary Sarcoidosis with Vision Loss and Hypopituitarism: Case Series and Literature Review, Pituitary, 19(2016):19â29.
[Crossref] [Google Scholar] [PubMed]
Copyright: © 2025 Lourdes de Fatima Ibanez Valdes, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.