Research Article: Journal of Drug and Alcohol Research (2025) Volume 14, Issue 11
New Aspects on Neurological Manifestations in Fabry Diseases, Drug Management and Comprehensive Review
Lourdes de Fatima Ibanez Valdes and Humberto Foyaca Sibat22*2Department of Neurology, Nelson Mandela Academic Hospital, Walter Sisulu University, South Africa
Humberto Foyaca Sibat2, Department of Neurology, Nelson Mandela Academic Hospital, Walter Sisulu University, South Africa, Email: humbertofoyacasibat@gmail.com
Received: 22-Sep-2025, Manuscript No. JDAR-25-171235; Editor assigned: 24-Sep-2025, Pre QC No. JDAR-25-171235 (PQ); Reviewed: 08-Oct-2025, QC No. JDAR-25-171235; Revised: 12-Dec-2025, Manuscript No. JDAR-25-171235 (R); Published: 19-Dec-2025, DOI: 10.4303/JDAR/236480
Abstract
Introduction: We searched the medical literature, following the guidelines outlined in the PRISMA statement. From 01st, January 1982 to 31st, July 2025, the authors searched the scientific databases, Scopus, Embassy, Medline, and PubMed Central using the following searches: “Fabry disease” OR “agalsidase alfa” OR “agalsidase beta” OR “lysosomal storage disease” OR “enzyme replacement therapy”, OR “migalastat” OR “α‐galactosidase A” OR “globotriaosylceramide” OR “globotriaosylsphingosine”.
Results: After screening the full‐text articles for relevance, n=2172 articles were identified through database searching. Included for the first screen n=1193. Records after duplicates removed n=923, record included for full‐test review n=270. Excluded by full‐test review n=101studies. Include publications for initial searches n=169. Additional reports after search es identified references (covering 171 studies) n=182. Identified publication with new proposal of pathogenesis included in final review and meta‐analysis n=0. No article proposing a new hypothesis for pathogenesis of FD responding to drug therapy was found when we searched. Therefore, meta‐analysis was not performed.
Conclusions: To the best of our knowledge, this is the first study proposing an update drug therapy program to control neurological manifestations of FD.
Keywords
Agalsidase alfa; Agalsidase beta; Efficacy; Effecveness; Fabry disease; Lysosomal storage disease; Migalastat; Enzyme replacement therapy
Introduction
Fabry Disease (FD), also named Anderson-Fabry disease, was first described by dermatologist Johannes Fabry and surgeon William Anderson in 1898. Fabry disease is also known as an uncommon, X-linked lysosomal sphingolipidosis disorder in which globotriaosylceramide is insufficiently metabolized because of reduced α-galactosidase A activity caused by leading to a remarkable accumulation of glycolipids-mainly globotriosyl ceramide (GL-3, GB3) and globotriaosylsphingosine (its dacylated product) in large variety of cells and in plasma along all over the body [1-4]. This enzyme mainly affects the heart, causing arrhythmias and cardiomyopathy, the kidneys, causing renal failure and proteinuria, the nervous system, leading to ischemic stroke, white matter lesions caused by Cerebral Small-Vessel Disease (CSVD) and neuropathic pain, and skin (angiokeratomas). The prevalence of FD is reported to be between 1 in 117,000 and 1 in 476,000.
Sometimes, FD is confirmed by a blood test measuring the activity of the affected enzyme (alpha-galactosidase). Still, genetic testing can also be used from time to time, particularly in females [1-4]. This inborn error may cause a systemic accumulation of glycosphingolipids in lysosomes and causes progressive and heterogeneous clinical manifestations such as cerebrovascular disease, renal failure, and cardiomyopathy, plus a significant diminishing life expectancy [5].
In Japan, ladies presenting FD face a remarkable lack of support from their physicians, community, emotional burdens, family planning, impact on children, inheritance and women’s mental health. The best procedure for confirming the diagnosis is the molecular genetic detection of a disease-causing mutation (in females) and the determination of reduced α-galactosidase A activity in leukocytes (in males) [6,7].
The main aim of this review was to find the available and emerging drug therapies for FD.
Materials and Methods
Search strategys
We searched the medical literature, following the guidelines outlined in the PRISMA statement. From 01st, January 1982 to 31st, July 2025, the authors searched the scientific databases, Scopus, Embassy, Medline, and PubMed Central using the following searches: “Fabry disease” OR “agalsidase alfa” OR “agalsidase beta” OR “lysosomal storage disease” OR “enzyme replacement therapy”, OR “migalastat” OR “α-galactosidase A” OR “globotriaosylceramide” OR “globotriaosylsphingosine”. Any disagreements between the reviewers involved in the literature search were resolved through discussion among the authors until a unanimous consensus was reached.
Selection criteria
The following manuscripts were included in the systematic review and meta-analysis:
Articles with detailed pathogenesis and/or drug therapy, Clinical features of FD, and demographic information.
Exclusion criteria were as follows:
• Inaccessibility to full text.
• Articles with unclear pathogenesis.
• Lack of relevant clinicopathological data;
• Non-original studies (i.e., editorials, letters, conference proceedings, book chapters).
• Animal model studies.
• Non-/Spanish/Portuguese/English studies.
The papers that were not thoroughly assessed were removed.
Data collection, extraction, and bias assessment
Relevant facts from eligible guides were extracted into an up to date Excel software program application. Extracted information covered the examine design, medical functions of sufferers, baseline demographics, duration of observeup, remedy info, consequences of hobby, funding data, and final feedback. publications had been summarized and categorized based totally on the subsequent categories: Agalsidase alfa investigations, agalsidase beta studies, blended or non-particular ERT or other treatment singlearm studies, and scientific trial studies. For every decided on ebook, a threat of bias evaluation changed into made the usage of the Joanna Briggs Institute (critical appraisal tick list) for research synthesis (for meta-analyses) and systematic opinions.
Data extraction and quality assessment
As mentioned before, all the included data were tabulated in an electronic Excel database. That information included pathogenesis, drug management, initial clinical presentation, evaluation after treatment, follow-up and status at the latest evaluation. The studies’ quality was categorized as good, poor, fair, or reasonable, in agreement with the National Institutes of Health criteria.
Statistical analysis
The main objective of this review was to evaluate whether reported pathogenesis/drug management differs significantly among different classic and novel therapies. Without a comprehensive reference for the total number of cases, the prevalence of responding to drug therapy was searched through a thorough review. Statistical analyses were performed using XLSTAT (add-on for Microsoft Excel, version 2021.4.1, Addinsoft SARL) and RStudio (version 4.3.1, https://www.rstudio.com/). Variations in continuous variables were assessed using the Mann-Whitney U-test. We presented descriptive statistics for continuous variables as median (95% Confidence Interval (95% CI)). All situations were evaluated using the Kaplan-Meier method to identify relevant prognosticators. A model of multivariable Cox proportional hazards with a priori selection of covariates was used to check for independent prognostic effects.
Results and Discussion
Of 2172 guides screened, 234 mentioned records on renal, cardiac, cerebrovascular, and sickness severity effects from 225 studies. maximum said investigations have been observational studies (n=150; 67%) and worried simplest adults (n=172; 74%).
1881 articles had been diagnosed from the electronic databases. an additional thirteen critical articles had been recognized in reference searches. After putting off duplicates and non-eligible research, 182 courses remained, overlaying a complete of 171 research. maximum research had been real-global, observational research (n=150; 67%); the ultimate research were case collection and reports (58%) and meta-analyses (n=1). maximum research blanketed handiest adults (67%). In research that suggested clinical outcomes, the number of protected sufferers ranged from twenty [8,9] to 2051 [10]. Seven studies covered over 525 patients [10-16].
233 research covered ERT with agalsidase alfa and/ or agalsidase beta, 44 studies covered remedy with migalastat, and 15 guides blanketed second-era ERT with pegunigalsidase alfa. Moreover, 31 guides tested mixed or non-precise ERT. Handiest 3 investigations as compared the outcomes of agalsidase beta and agalsidase alfa [17-19]. 24 courses assessed the effects of switching among specific treatment options, however the consequences of the transfer had been not mentioned as outcomes [20-27].
However, no publications regarding new hypotheses on the pathogenesis of FD responding completely to drug therapy were found. No articles were selected for full-text review for the meta-analysis because they did not present a new hypothesis on FD responding to drug therapy.
Series description and differences among groups
Median age was 14.7 (range 6-79) with significant differences between age groups (p<0.001). We did not find remarkable variations in gender (p=0.052), although males presenting with FD were noticeably more frequent and slightly more prevalent Figure 1.
Figure 1: The 3 transcripts arising from the pathogenic GLA variant c.639+5G>C. The location of c.639+5G>C pathogenic variant is on the fifth nucleotide of the intron 4 splice donor consensus sequence. The corresponding pre-mRNA is spliced into the wild-type mRNA transcript, containing the 7 exons of the GLA gene, along with 2 abnormally spliced isoforms: alternative transcript 1, lacking the entire exon 4 (r.570_661del p.Gly183Alafs18*), and alternative transcript 2, lacking only the last 34 base pairs of the exon 4 (r.627_661del p.Cys202Serfs18*). We hypothesize that the c.639+5G>C transversion compromises the stability of the spliceosome binding to the pre-mRNA sequence in that location and that the presence of the 5-nucleotide sequence c.605_609 (gtgag), which is identical to consensus sequence in the exon 4
Brief comments on FD-associate pain
One of the most common and early manifestations of FD is pain, even from childhood, affecting the quality of life of patients, also causing an associated neuropsychiatric symptom [28].
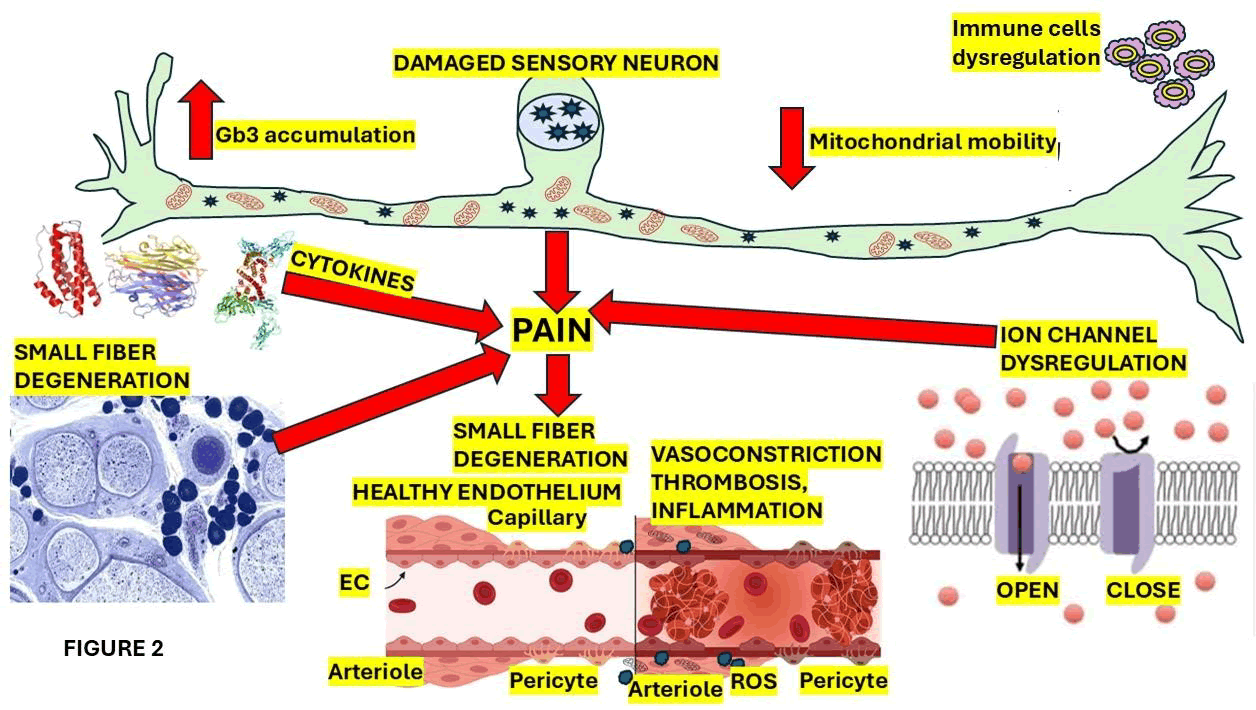
Some of the most crucial elements involved in the pain mechanism are represented in Figure 2.
Figure 2: Show a graphical representation of the affected sensory neuron cells and the thinly myelinated A-delta apart from unmyelinated C nerve fibers (small nerve fibers). Because peripheral nerve damage of the large nerve fibers in terms of polyneuropathy with a sensorimotor clinical phenotype is quite uncommon. In FD cases presenting small fiber neuropathy, the clinical phenotype is characterized by decrease heat sensation while sensory profiles of FD cases mainly show an impairment in cold feeling. Hypo to anhidrosis is the principal complaint of these patients that may also trigger/enhance heat intolerance. FD patients may further complain of gastrointestinal symptomatology secondary to autonomic dysfunction of small caliber nerve fibers, leading to dyspepsia, dysmotility, diarrhea and cardiac dysautonomia
Pain associated with FD is most often episodic and triggerable by heat, physical activity, and fever. In most cases, the pain has a character of burning pain (neuropathic pain) due to impairment of small nerve fibres, usually located in the toes, soles, palms, and fingertips. However, it can be present in any region of the body, including abdominal pain [28]. There are four pain subgroups, such as:
• Pain attacks (most common), which are characterised by intense and sudden pain upon triggers and typically persist until the trigger is removed.
• Pain crises are severe, radiating pain over the body. Its highest intensity requires urgent hospitalisation, and it is not related to or preceded by triggering factors, being relatively refractory to first-line analgesics.
• Evoked pain is usually present after hot or cold contact.
• Chronic pain, like persistent pain, is rare in FD.
Globotriaosylsphingosine (lyso-Gb3) is elevated in the plasma of FD patients, where pain is attributed to high levels of intracellular calcium, leading to hyperexcitation of nociceptors [28].
Based on the current Delphi consensus recommendations, duloxetine and pregabalin can be prescribed as a first-line analgesic medication for the management of neuropathic pain in FD. Topical patches of lidocaine and capsaicin can be placed on the correct dermatome as a second-line analgesic therapy. Amitriptyline can also be used as a firstline analgesic medication [29].
Recently, Nunez et al. [30] reported that the most relevant pathophysiological impact of Gb3 storage is on cardiomyocytes, glomerular podocytes, the vascular endothelial and arterial smooth muscle cells, renal tubular cells, neurons of the CNS and somatic and autonomic peripheral nervous systems. They also documented that the deacylated metabolite of Gb-3, globotriaosylsphingosine (lysoGb3), plays a crucial role in the pathogenesis of FD.
The sustainable reason why FD is usually more aggressive in the early stages of male patients compared with female ones is due to the location of the GLA gene on the X chromosome (Xq22.1) and the X-linked inheritance.
FD can be classified into type 1 (Classic) due to impaired α-galactosidase expression associated with GLA variants functional enzymatic expression determining the extension. The remaining enzymatic expression is good enough to impede Gb3 deposition in the blood vessels, including CV. The clinical manifestation related to the peripheral nerve’s involvement is characterised by the neuropathic pain, commonly chronic in evolution, classically reported as tingling, burning, or nagging, affecting symmetrically the soles of the feet and palms of the hands (“acroparaesthesia”). Some types of attacks of excruciating pain (“pain crises”) are triggered by physical activity, stress, fever, and fatigue [30].
GLA oversees the catalytic process of hydrolysing terminal, nonreducing α-galactose residues in α-galactosidase. Abnormal α-galactosidase expression leads to accumulation of undegraded neutral glycosphingolipids, mainly globotriaosylceramide (Gb3), in the lysosomes of many cells of different organs [30,31].
FD has been well documented as a subgroup of autoinflammatory diseases in which the p65/p50 heterodimer (a novel variant of p65) is the most commonly present as a family of NF-κB involved in the pathophysiology of inflammation, and being the Glucocorticoid Receptor (GR), the most remarkable physiological antagonist, as has been reported by Biddeci and collaborators. These authors studied the potential role of p65 iso5 in the inflammation of patients presenting with FD and the Peripheral Blood Mononuclear Cells (PBMCs), the protein expression and the level of p65 iso5 mRNA in more than one hundred 100 cases with FD, and they found the lowest p65 iso5 mRNA and protein expression levels when they compared the FD group with controls, confirming that p65 iso5 is highly involved in the inflammatory process of FD [32].
Natale et al. studied 40 adult patients presenting with FD, assessing their prevalence of stroke and cognitive decline related to heart and renal involvement, and the real value of serum neurofilament light chain (NfL) levels as an indicator of White Matter Lesions (WMLs) being a biomarker of CSVD in FD. These investigators found that mild-moderate CSVD is a typical brain “signature” in FD cases, brain microvascular damage is correlated with mild cognitive impairment, and cardiac and renal involvement correlate with WML load. However, only kidney involvement is predictive of CNS injury [33].
This year, at the Stroke Centre of Torre Cárdenas University Hospital in Almería (Spain), a group of investigators studied the clinical manifestations of the central and peripheral nervous system seen in FD. They also assessed the frequency of association between FD and stroke, reporting a 4% of cryptogenic stroke. This study revealed a remarkably lower percentage than that expected.
Polyneuropathy is the most frequent type of presentation (80%) in FD [34]. The same authors found that some cerebrovascular diseases, like Transient Ischemic Attacks (TIAs)/ ischemic strokes, are quite frequent (25%) in men aged 25-44 years, although less frequent than haemorrhagic stroke, vascular dementia, and cervical artery dissection with FD, which has been supported by others [35].
Magnetic resonance imaging is the ideal investigation for identifying abnormalities in FD based on its capacity to show nonspecific signs like ectasia and elongation of the basilar artery [36], extensive white matter lesions [37], or the pulvinar sign, which should be considered in the clinical context of the case [38].
Leng et al. investigated vestibular involvement in FD in a cohort study of 37 Chinese FD patients a few months ago. They reported visuo-oculomotor disturbances in 81.1% of the cases and vestibulo-oculomotor dysfunctions in 40.9% of the total series, concluding that the incidence of extensive central and peripheral vestibular dysfunctions is high and strongly related to α-Gal A activity, lyso-Gb3, the disease burden, and the deposition of substrates in vestibular organs [39].
This year, investigators from the Copenhagen Hearing and Balance Centre, Department of Nephrology and Endocrinology, and Institute of Clinical Medicine in Copenhagen, Denmark, conducted a study on dizziness/ balance issues in Fabry patients, finding a high prevalence due to a central cause based on an abnormal optokinetic test. The authors concluded that dizziness/balance issues might be caused by polypharmacy present in all eight examined patients [40].
Brief comments on the current available therapy for FD
Recently, Guigliani et al. established that in Latin America, there are specific limitations for the therapy of FD due to the delay of access to diagnostic tools and the high cost of the current three therapeutic options [41].
Therapy for FD includes the augmentation or replacement of α-Gal A function [42-44].
On the other hand, new investigations on FD therapy continue to be developed under clinical trials, including gene therapy to manage the genetic defect leading to substrate reduction therapy and α-Gal A deficiency, a novel approach to modulate [45,46].
Some of the most relevant therapeutic procedures used to treat FD are represented in Figure 3.
Figure 3: Molecular targets for Fabry disease therapeutics
Contemporary healing alternatives include hooked up treatments which includes one chaperone treatment (migalastat), enzyme replacement treatment plans (agalsidase α, agalsidase β) and a currently approved enzyme substitute therapy targeting pegunigalsidase α. Then again, new drug therapies are being brought, inclusive of mRNA remedy, substrate discount therapy, and genetic therapy. See discern X. As formerly noted, the mutation in the GLA gene (X chromosome) encoding α-galactosidase A (α-GALA) ends in a progressive accumulation of its substrate: Globotriaosylceramide (Gb3) and its nicely-acknowledged substrate globotriaosylsphingosine (lyso-Gb3), in several cellular types, which include cardiomyocytes, peripheral neurons, endothelial cells, and renal cells [47,48]. As represented in discern X, therapeutic selections include ERT and oral chaperone treatment. The class of ERT consists of agalsidase α, agalsidase β and pegunigalsidase α, recombinant α-Gal A proteins, which can be notably powerful in controlling the medical manifestations of FD and slowing/preventing ailment progression [49].
The primary pharmacological function of migalastat is to stabilise the residual endogenous enzyme, correcting its misfolding to restore the degradation of glycolipids [50].
To evaluate the benefits of migalastat, it is recommended to measure the AGAL activity in treated patients who exhibit highly elevated AGAL expression and demonstrate appropriate compliance [51]. concerning the substrate reduction therapy (discern 1), it is designed to diminish the elaboration of metabolic elements that can’t be degraded because of the inherent enzymatic deficiency that includes Venglustat and Lucerastat, that have the capability to inhibit Glucosylceramide Synthase (GCS) to modulate the wide variety of molecules not degradable because of a loss of α-GalA enzyme [52].
Another modality of remedy for FD is gene therapy, that is designed to direct the remedy towards circulating hematopoietic cells, correcting the target cells, and using a recombinant vector capable of transducing cells to supply a extensive amount of α-galactosidase A intracellularly and of their secretions [48].
The messenger RNA-based treatment aims to enhance endogenous protein synthesis, thereby improving the glycosylation, conformational maturation, and distribution of α-GAL A in cells [53]. Other investigators have been introducing a novel variant to reduce intra-lysosomal storage (in vitro) by enhancing the eflux of stored molecules from the lysosomal vesicle, leading to better chances of subsequent expulsion from the cell [48].
Neurologic symptomatic treatment
Ischemic stroke complications require cerebrovascular prophylaxis with aspirin or clopidogrel (antithrombotic agents) for secondary prevention. Neuropathic pain is treated with some anti-epileptic drugs (carbamazepine, gabapentin or pregabalin), and opioid agonists in cases presenting intense pain crises. To prevent pain exacerbations and avoid temperature extremes, lifestyle modifications, such as maintaining proper hydration, are strongly suggested [48].
Non-steroidal anti-inflammatory drugs can cause nephrotoxicity side effects, and on top of that, their analgesic effect in neuropathic pain seems to be low. Therefore, they should be prescribed with caution.
If the mutation is amenable, (agalsidase alfa, pegunigalsidase alfa, every 2 weeks intravenously) or chaperone therapy (migalastat, one capsule orally every second day [54].
Brief comments on enzyme replacement therapy
An ideal therapy for FD based on the results reported by many publications, which have confirmed the relevant ameliorative effect of Agalactosidase-α (ALTA-a) on renal and cardiac function in cases, despite the scant and limited pharmacoeconomic investigations. Reporting that ERT therapy is an economically viable choice from the perspective of the public health system. It will support interested organisations in their decision-making process, and their safety, excellent efficacy, and affordability of ERT to be a dominant therapy for FD [55].
Parini et al. established that administering Agalsidase Alfa ERT in childhood or early adulthood, as opposed to starting it in later adulthood, provides a significant benefit to the kidney and heart in FD [56]. The main goal of ERT is the intravenous infusion of a recombinant protein.
The pharmacokinetic mechanism of ERT via intravenous management of recombinant α-galactosidase A (α-Gal A) is to reduce the lysosomal accumulation of Gb3 [48]. ERT consists of agalsidase β (Fabrazyme, Sanofi Genzyme), administered at a dose of 1.0 mg/kg biweekly and agalsidase α (Takeda, Replagal), prescribed at a dose of 0.2 mg/kg biweekly. each agalsidase use the Mannose-6-Phosphate Receptor (M6PR), which identifies the enzyme’s Mannose 6-Phosphate (M6P) residues, to enter lysosomes and modulate their healing function.
Pegunigalsidase α is a singular ERT which has supplied stability and better half-life way to PEGylation.
In advanced FD patients, agalsidase β has been established to gradual the development of renal, and cerebrovascular complications such as increasing height systolic pressure rate, maximal myocardial thickness, mitigation of left ventricular hypertrophy, reduction of total left ventricular mass, and myocardial T2 relaxation time; apart from to facilitate the clearance of Gb3 deposits and slow the FD development [48,54].
The advantages of agalsidase α have also been broadly documented, which consist of slowing the decline in kidney feature and offering a later onset of cardiac, renal, and neurological complications. It additionally provides an improvement in cardiac mass with a reduction in left ventricular mass after six months of therapy, a lower in myocardial Gb3 content and a sustainable behind schedule mortality technique.
Sugiura and collaborators suggested a case providing surprising arterial hypotension after six years of treatment with agalsidase α for FD and recommended careful monitoring throughout ERT [57].
Pegunigalsidase α (PRX-102) is generated in the cells of the tobacco plant as a chemically cross-linked and PEGylated α-Gal A with the capacity to enhance the enzyme’s balance and lengthen its half-lifestyles. PRX-102 has a remarkably longer plasma half-life of 80 h, compared to agalsidase β (less than one hour), with the capacity to reduce Gb3 by 84% in the renal system by reducing the formation of anti-drug antibodies and to reverse existing ones, promoting immune tolerance, leading to less adverse immune reactions and making the treatment more effective. Although some cases of glomerulonephritis have been reported [48,54].
it’s a potent inhibitor of α-GAL A with the capacity to increase enzyme activity for specific GLA variants [48]. On the other hand, it has some disadvantages, such as producing anti-drug antibodies and the inability to pass across the BBB efficiently. However, it has been recommended as an alternative therapy for FD, as a chaperone drug that binds to a particular molecule of sugar inside the catalytic process. Increasing and reducing Gb3 levels in most tissues, including the brain [48,54] Another ERT used for the drug therapy of FD is produced in a non-phosphorylated form, which has shown significant efficacy in animal models utilising the mannose receptor in endothelial cells, macrophages, and renal cells.
Apart from novel gene therapy, messenger RNA therapy, and substrate reduction therapy, forthcoming new therapeutic choices may include vesicle-packaged enzyme replacement therapy [58].
Comments on new emerging therapies
Novel drugs are being recommended in current investigations, such as second-generation enzymes, substrate reduction Table 1.
|
Administration |
Advantages |
|
|
Lucerastat |
100, 300, 500, 1,000 mg once daily |
Oral administration |
|
200, 500, 1,000 mg b.i.d. |
||
|
Moss-galactosidase alfa |
Repeated administration |
Safety and tolerability |
|
Gene therapy |
Single administration |
Prolonged treatment |
|
mRNA-based therapy |
Repeated administration |
No immunogenicity |
|
Venglustat |
15 mg once daily |
Oral administration |
Table 1: Characteristics of emerging therapies
It is mandatory to prescribe carefully the exact dosage of the drug, because complete inhibition of a single enzyme can disrupt cellular homeostasis, causing dysfunctional cell growth/differentiation and programmed cell death.
To make inhibition of Glucosylceramide Synthase (GCS) as part of the therapy for FD, two new products were made:
• Lucerastat (Idorsia Pharmaceuticals).
• Venglustat (Sanofi Genzyme), which we will comment briefly below.
Lucerastat, also known as N-butyldeoxygalactonojirimycin, derived from galactose and administered orally.
While venglustat is designed to inhibit GCS, it can decrease the capacity to produce one precursor of glycosphingolipids, such as glucosylceramide. When given 15 mg orally once daily, it can lower levels of plasma globotriaosylsphingosine and key glycosphingolipid pathway markers, thereby reducing neuropathic and abdominal pain.
The main aid of gene and mRNA-based therapies is to provide a large therapy plan to address all damaged tissues and cells in patients with FD reducing in plasma even though their effect is transitory and repeated administration is required.
Lentiviral-based approach
Lentiviral-based approach for FD is based on subsequent re-implantation of transduced cells and ex vivo transduction of hematopoietic stem cells. Despite its ability to trigger leukemogenesis, In some anecdotal cases of T-cell leukaemia, the lyso-Gb3 levels in plasma and urine decreased in most patients [58].
AAV-based approach
Adeno-associated virus-based vectors are one of the more effective approaches in gene therapy because they exhibit lower immunogenicity, despite AAV persisting in the infected person for life after infection. After all, gene transfer with AAV vectors is well-tolerated by people without immune reactions. However, some neurological injuries, such as cerebral T2 hyperintensities and loss of dorsal root ganglia, have been reported [59]. On the other hand, AAV vectors cause direct T-cell-mediated immune response and transduction, leading to virus-specific antibody formation, triggering some forthcoming inflammatory reactions [59]. Therefore, patients must be tested for AAV-neutralising antibodies (pre-existing) before administering any AAVbased gene therapies. Other investigators have suggested plasmapheresis (with or without immune adsorption), proteasome inhibition (bortezomib, in vivo IgG destruction (imlifidase) and B-cell depletion (rituximab) on initial or repeated AAV transduction [59].
Neutralizing ADAs
Around 40–70% of all FD’s male patients, with the absence of endogenous AGAL expression on ERT, present IgG antibodies against the infused enzymes. These antibodies provide a high cross-reactivity towards agalsidase beta and alpha, decreasing the therapeutic efficacy and leading to enzyme inhibition [60]. ADAs against agalsidase beta or agalsidase alfa lead to a lower affinity and a lower inhibitory capacity against pegunigalsidase alfa [61].
The therapeutic plan to prevent or diminish ADAs in FD should include immunosuppressive treatment of patients [62].
Brief comments on some treatment pitfalls
Many years ago, several authors reported that some infused enzymes can be recognised as foreign and induce a corresponding immune response, leading to the formation of antibodies against the enzyme [63].
Nevertheless, Infusion-Associated Reactions (IARs) are seen more often in ERT-Naïve cases with FD soon after initialisation of ERT and their clinical features commonly are related to fever and/or chills, which respond appropriately to antihistamines, corticosteroids and reduced infusion rate [64].
Furthermore, other milder symptoms like facial flushing or swelling, headache and aching limbs, urticarial skin lesions, plus more severe reactions such as throat and chest tightness, shortness of breath, life-threatening anaphylactic reactions and hypotension were also reported [65-68].
From our review of the medical literature, we found that 67% of patients treated with agalsidase beta and 24% of cases treated with agalsidase alfa had these adverse reactions. At the same time, 22% of patients treated with pegunigalsidase alfa reported the same anaphylactoid (non-IgE-mediated) reactions, especially those who were IgE-positive (anaphylactic reaction) to recombinant AGAL and cases that developed anti-agalsidase IgG antibodies [69,70].
Conclusion
The final conclusions for this review are summarized:
• Male patients with Fabry’s disease present from childhood with burning, lancinating pain (sometimes acroparaesthesia) and hypohidrosis.
• Female patients with Fabry’s disease are phenotypically more heterogeneous than are male patients, but can also present with severe symptoms.
• Stroke appears in early life, with male patients presenting at a lower age than female patients, although the frequency is higher among females.
• Misdiagnosis and delays in diagnosis are major problems; it is important for neurologists to consider a diagnosis of Fabry’s disease in patients presenting with neuropathic pain and premature idiopathic stroke.
• Enzyme replacement therapy is essential before irreversible organ damage occurs, and supportive therapy is necessary to prevent stroke, to treat neuropathic pain and to increase quality of life
• Genetic counselling is essential, as is a multidisciplinary approach to managing patients with Fabry’s disease. Enzyme Replacement Therapy (ERT) is the ideal treatment for FD of the manuscript.
Acknowledgment
To thanks to Prof Thozama Dubula for his encouragement.
Ethics Statement
This review does not require ethical approval.
Patient Privacy
All patientâidentifying information has been removed to ensure anonymity.
References
- D.P. Germain, Fabry disease, Orphanet J Rare Dis, 5(2010):30-79.
[Crossref] [Google Scholar] [PubMed]
- A. Jovanovic, E. Miller-Hodges, F. Castriota, O. Evuarherhe, O. Ayodele, et al. Clinical Efficacy and Real-World Effectiveness of Fabry Disease Treatments: A Systematic Literature Review, J Clin Med, 14(2025):5131.
[Crossref] [Google Scholar] [PubMed]
- R. Giugliani, J. Politei, A. Martins, N. Murillo, P. Rozenfeld, et al. Expert review in diagnostic, therapeutic and follow-up of Fabry disease in Latin America based on patient care standards, Mol Genet Metab Rep, 43(2025):101218.
[Crossref] [Google Scholar] [PubMed]
- C. Tognola, G. Ruzzenenti, A. Maloberti, M. Varrenti, P. Mazzone, et al. Anderson-Fabry disease: an overview of current diagnosis, arrhythmic risk stratification, and therapeutic strategies, Diagnostics, 15(2025):139.
[Crossref] [Google Scholar] [PubMed]
- K.D. MacDermot, A. Holmes, A.H. Miners, Anderson-Fabry disease: Clinical manifestations and impact of disease in a cohort of 98 hemizygous males, J Med Genet, 38(2001):750-760.
[Crossref] [Google Scholar] [PubMed]
- M. Kobayashi, I. Kaku, N. Goto, M. Tsuchiya, N. Sakai, Exploring the burdens of women living with Fabry disease in Japan: A patient survey of 62 respondents, Mol Genet Metab Rep, 43(2025):101231.
- M.J. Page, J.E. McKenzie, P.M. Bossuyt, I. Boutron, T.C. Hoffmann, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ, 372(2021):n71.
[Crossref] [Google Scholar] [PubMed]
- G. Bierer, D. Balfe, W.R. Wilcox, Z. Mosenifar. Improvement in serial cardiopulmonary exercise testing following enzyme replacement therapy in Fabry disease, J Inherit Metab Dis, 29(2006):572–579.
[Crossref] [Google Scholar] [PubMed]
- D. Bodensteiner, C.R. Scott, K.B. Sims, G.M. Shepherd, R.D. Cintron, et al. Successful reinstitution of agalsidase beta therapy in Fabry disease patients with previous IgE-antibody or skin-test reactivity to the recombinant enzyme, Genet Med, 10(2008):353-358.
[Crossref] [Google Scholar] [PubMed]
- D. Hughes, A. Linhart, A. Gurevich, V. Kalampoki, D. Jazukeviciene, et al. Prompt initiation of agalsidase alfa therapy is associated with improved cardiovascular and renal outcomes in the Fabry outcome survey (FOS), Mol Genet Metab, 129(2020):S77.
- M. Beck, D. Hughes, C. Kampmann, G. Pintos-Morell, U. Ramaswami, et al. Long-term outcomes with agalsidase alfa enzyme replacement therapy: Analysis using deconstructed composite events, Mol Genet Metab, Rep,14(2018):31-35.
- S. Feriozzi, A. Linhart, U. Ramaswami, V. Kalampoki, A. Gurevich, et al. Effects of baseline left ventricular hypertrophy and decreased renal function on cardiovascular and renal outcomes in patients with Fabry disease treated with agalsidase alfa: A Fabry outcome survey study, Clin Ther, 42(2020):2321-2330.
[Crossref] [Google Scholar] [PubMed]
- R.J. Hopkin, G.H. Cabrera, J.L. Jefferies, M. Yang, E. Ponce, et al. Clinical outcomes among young patients with Fabry disease who initiated agalsidase beta treatment before 30 years of age: An analysis from the Fabry registry, Mol Genet Metab,138(2023):106967.
[Crossref] [Google Scholar] [PubMed]
- D. Hughes, A. Linhart, A. Gurevich, V. Kalampoki, D. Jazukeviciene, et al. Prompt agalsidase alfa therapy initiation is associated with improved renal and cardiovascular outcomes in a Fabry outcome survey analysis, Drug Des Dev Ther,15(2021):3561–3572.
[Crossref] [Google Scholar] [PubMed]
- A. Ortiz, A. Abiose, D.G. Bichet, G. Cabrera, J. Charrow, et al. Time to treatment benefit for adult patients with Fabry disease receiving agalsidase beta: Data from the Fabry Registry, J Med Genet, 53(2016):495-502.
- R. Parini, G. Pintos-Morell, J.B. Hennermann, T.R. Hsu, N. Karabul, et al. Analysis of renal and cardiac outcomes in male participants in the Fabry outcome survey starting agalsidase alfa enzyme replacement therapy before and after 18 years of age, Drug Des Dev Ther,14(2020):2149-2158.
- S.M. Sirrs, D.G. Bichet, R.M. Iwanochko, A. Khan, S. Doucette, et al. Differential effects of agalsidase alfa and agalsidase beta in Fabry outcomes: 10 year outcomes from the Canadian Fabry disease initiative, J Inherit Metab Dis, 41(2018):S188.
- A.C. Vedder, G.E. Linthorst, G. Houge, J.E. Groener, E.E. Ormel, et al. Treatment of Fabry disease: Outcome of a comparative trial with agalsidase alfa or beta at a dose of 0.2 mg/kg, PLoS One, 2(2007):e598.
[Crossref] [Google Scholar] [PubMed]
- M. Arends, M. Biegstraaten, C. Wanner, S. Sirrs, A. Mehta, et al. Agalsidase alfa versus agalsidase beta for the treatment of Fabry disease: An international cohort study, J Med Genet, 55(2021):351-358.
[Crossref] [Google Scholar] [PubMed]
- G. Cabrera, J. Politei, N. Antongiovani, H. Amartino, Effectiveness of enzyme replacement therapy in Fabry disease: Long term experience in Argentina, Mol Genet Metab Rep, 11(2017):65-68.
[Crossref] [Google Scholar] [PubMed]
- M. Arends, M. Biegstraaten, D.A. Hughes, A. Mehta, P.M. Elliott, et al. Retrospective study of long-term outcomes of enzyme replacement therapy in Fabry disease: Analysis of prognostic factors, PLoS One, 12(2017):e0182379.
[Crossref] [Google Scholar] [PubMed]
- M. Arends, F.A. Wijburg, C. Wanner, F.M. Vaz, A.B. van Kuilenburg, et al. Favourable effect of early versus late start of enzyme replacement therapy on plasma globotriaosylsphingosine levels in men with classical Fabry disease, Mol Genet Metab, 121(2017):157-161.
[Crossref] [Google Scholar] [PubMed]
- S. van der Veen, S. Körver, A. Hirsch, C. Hollak, F. Wijburg, et al. Early start of enzyme replacement therapy in pediatric male patients with classical Fabry disease is associated with attenuated disease progression, Mol Genet Metab, 135(2022):163-169.
[Crossref] [Google Scholar] [PubMed]
- A. Nowak, G. Koch, U. Huynh-Do, M. Siegenthaler, H.P. Marti, et al. Disease progression modeling to evaluate the effects of enzyme replacement therapy on kidney function in adult patients with the classic phenotype of Fabry disease, Kidney Blood Press Res, 2(2017):1-15.
- K. Wyatt, W. Henley, L. Anderson, R. Anderson, V. Nikolaou, et al. The effectiveness and cost-effectiveness of enzyme and substrate replacement therapies: A longitudinal cohort study of people with lysosomal storage disorders, Health Technol Assess, 16(2012):1-543.
- L.J. Anderson, K.M. Wyatt, W. Henley, V. Nikolaou, S. Waldek, et al. Longâterm effectiveness of enzyme replacement therapy in Fabry disease: Results from the NCSâLSD cohort study, J Inherit Metab Dis, 37(2014):969-978.
[Crossref] [Google Scholar] [PubMed]
- C.V. Madsen, H. Bundgaard, Å.K. Rasmussen, S.S. Sørensen, J.H. Petersen, et al. Echocardiographic and clinical findings in patients with Fabry disease during longâterm enzyme replacement therapy: A nationwide Danish cohort study, Scand Cardiovasc J, 51(2017):207–216.
- V.K. Medala, N. Üçeyler, Neuropathy and pain in Fabry disease, Rare Dis Orphan Drugs J, 3(2024):20.
- K.M. Stepien, A. Broomfield, D. Cole, Management of pain in Fabry disease in the UK clinical setting: Consensus findings from an expert Delphi panel, Orphanet J Rare Dis, 18(2023):203.
- J.P.L. Nunes, R. SoaresâdosâReis, M.S. Faria, E. Martins, T. Pinho, et al. Renal, cardiac, and neurologic disease in a patient with Fabry disease, hemizygous for the c.639+5G>C intronic variant in the galactosidase alpha (GLA) gene, Porto Biomed J, 10(2025):e291.
[Crossref] [Google Scholar] [PubMed]
- J.P. Oliveira, S. Ferreira, Multiple phenotypic domains of Fabry disease and their relevance for establishing genotypeâphenotype correlations, Appl Clin Genet, 12(2019):35-50.
[Crossref] [Google Scholar] [PubMed]
- G. Biddeci, G. Spinelli, P. Colomba, G. Duro, M. Anania, et al. Fabry disease and inflammation: Potential role of p65 iso5, an isoform of the NFâκB complex, Cells, 14(2025):230.
[Crossref] [Google Scholar] [PubMed]
- D. Di Natale, S. Rossi, G. Dalla Zanna, A. Funcis, T.F. Nicoletti, et al. Prevalence and clinical correlates of cerebrovascular alterations in Fabry disease: A crossâsectional study, Brain Sci, 15(2025):166.
- M.L. RuizâFranco, B. VélezâGómez, P. MartínezâSánchez, R. GarófanoâLópez, C. GómezâNavarro, et al. Cryptogenic strokes and neurological symptoms of Fabry disease, Front Neurol, 16(2025):1529267.
- M. Ranieri, G. Bedini, E.A. Parati, A. Bersano, Fabry disease: Recognition, diagnosis, and treatment of neurological features, Curr Treat Options Neurol, 18(2016):33.
[Crossref] [Google Scholar] [PubMed]
- A. Fellgiebel, I. Keller, D. Marin, M.J. Müller, I. Schermuly, et al. Diagnostic utility of different MRI and MR angiography measures in Fabry disease, Neurology, 72(2009):63–68.
- A. Fellgiebel, M. Mazanek, C. Whybra, M. Beck, R. Hartung, Pattern of microstructural brain tissue alterations in Fabry disease: A diffusionâtensor imaging study, J Neurol, 253(2006):780–787.
[Crossref] [Google Scholar] [PubMed]
- A.P. Burlina, R. Manara, C. Caillaud, J.P. Laissy, M. Severino, et al. The pulvinar sign: Frequency and clinical correlations in Fabry disease, J Neurol, 255(2008):738–744.
[Crossref] [Google Scholar] [PubMed]
- Fabry disease
- A.B. Johansen, U. FeldtâRasmussen, M. Klokker, Dizziness in Fabry disease, Biomedicines, 13(2025):249.
[Crossref] [Google Scholar] [PubMed]
- R. Giugliani, J. Politei, A. Martins, N. Murillo, P. Rozenfeld, et al. Expert review in diagnostic, therapeutic and followâup of Fabry disease in Latin America based on patient care standards, Mol Genet Metab Rep, 43(2025):101218.
[Crossref] [Google Scholar] [PubMed]
- European Medicines Agency, Fabrazyme, Summary of Product Characteristics 2024, (2025).
- Food and Drug Administration, Fabrazyme, Prescribing Information 2024, (2025).
- European Medicines Agency, Replagal, Summary of Product Characteristics 2022, (2025).
- M. Lenders, E. Brand, Fabry disease: The current treatment landscape, Drugs, 81(2021):635–645.
[Crossref] [Google Scholar] [PubMed]
- S.J. van der Veen, C.E.M. Hollak, A.B.P. van Kuilenburg, M. Langeveld, Developments in the treatment of Fabry disease, J Inherit Metab Dis, 43(2020):908–921.
[Crossref] [Google Scholar] [PubMed]
- F. Amodio, M. Caiazza, E. Monda, An overview of molecular mechanisms in Fabry disease, Biomolecules, 12(2022):1460.
[Crossref] [Google Scholar] [PubMed]
- M. Giliberti, S. Robles, L. Gesualdo, The landscape of current and future therapeutic opportunities for Fabry disease, J Transl Genet Genom, 8(2024):340–354.
- D.G. Bichet, R. Torra, E. Wallace, D. Hughes, R. Giugliani, et al. Longâterm followâup of renal function in patients treated with migalastat for Fabry disease, Mol Genet Metab Rep, 28(2021):100786.
[Crossref] [Google Scholar] [PubMed]
- F.K. Johnson, P.N. Jr. Mudd, A. Bragat, M. Adera, P. Boudes, Pharmacokinetics and safety of migalastat HCl and effects on agalsidase activity in healthy volunteers, Clin Pharmacol Drug Dev, 2(2013):120–132.
[Crossref] [Google Scholar] [PubMed]
- D.G. Bichet, R.J. Hopkin, P. Aguiar, S.R. Allam, Y.H. Chien, et al. Consensus recommendations for the treatment and management of patients with Fabry disease on migalastat: A modified Delphi study, Front Med Lausanne, 10(2023):1220637.
[Crossref] [Google Scholar] [PubMed]
- T.M. Cox, Innovative treatments for lysosomal diseases, Best Pract Res Clin Endocrinol Metab, 29(2015):275–311.
[Crossref] [Google Scholar] [PubMed]
- X. Zhu, L. Yin, M. Theisen, J. Zhuo, S. Siddiqui, et al. Systemic mRNA therapy for the treatment of Fabry disease: preclinical studies in wildâtype mice, Fabry mouse model, and wildâtype nonâhuman primates, Am J Hum Genet, 104(2019):625–637.
[Crossref] [Google Scholar] [PubMed]
- I. Keyzor, A.M. Martins, S.K. Uçar, H. Yamakawa, Y.H. Chien, et al. A multiâcountry time and motion study to describe the experience and burden associated with the treatment of Fabry disease with enzyme replacement therapy with agalsidase alfa and agalsidase beta, Orphanet J Rare Dis, 20(2025):419.
[Crossref] [Google Scholar] [PubMed]
- Y. Huang, H. Yuan, Z. Huang, Costâeffectiveness analysis of enzyme replacement therapy for the treatment of Chinese patients with Fabry disease: A Markov model, Front Pharmacol, 16(2025):1546018.
[Crossref] [Google Scholar] [PubMed]
- R. Parini, G. PintosâMorell, J.B. Hennermann, T.R. Hsu, N. Karabul, et al. Analysis of renal and cardiac outcomes in male participants in the Fabry outcome survey starting agalsidase alfa enzyme replacement therapy before and after 18 years of age, Drug Des Devel Ther, 14(2020):2149–2158.
[Crossref] [Google Scholar] [PubMed]
- T. Sugiura, R. Muto, T. Amano, F. Kamiya, Y. Sato, et al. Unexpected hypotension in a female patient with Fabry disease: Switching from agalsidase α to β after longâterm ERT, Intern Med, 64(2025):2369–2374.
[Crossref] [Google Scholar] [PubMed]
- M. Lenders, E.R. Menke, E. Brand, Progress and challenges in the treatment of Fabry disease, BioDrugs, 39(2025):517–535.
[Crossref] [Google Scholar] [PubMed]
- H.C.J. Ertl, Mitigating serious adverse events in gene therapy with AAV vectors: Vector dose and immunosuppression, Drugs, 83(2023):287–298.
[Crossref] [Google Scholar] [PubMed]
- M. Lenders, J. Stypmann, T. Duning, B. Schmitz, S.M. Brand, et al. Serum-mediated inhibition of enzyme replacement therapy in Fabry disease, J Am Soc Nephrol, 27(2016):256–264.
[Crossref] [Google Scholar] [PubMed]
- M. Lenders, S. Pollmann, M. Terlinden, E. Brand, Pre-existing anti-drug antibodies in Fabry disease show less affinity for pegunigalsidase alfa, Mol Ther Methods Clin Dev, 26(2022):323–330.
[Crossref] [Google Scholar] [PubMed]
- Z.B. Kazi, A.K. Desai, K.L. Berrier, R.B. Troxler, R.Y. Wang, et al. Sustained immune tolerance induction in enzyme replacement therapy-treated CRIM-negative patients with infantile Pompe disease, JCI Insight, 2(2017):e94328.
[Crossref] [Google Scholar] [PubMed]
- G.E. Linthorst, C.E.M. Hollak, W.E. Donker-Koopman, A. Strijland, J.M.F.G. Aerts, Enzyme therapy for Fabry disease: Neutralizing antibodies toward agalsidase alpha and beta, Kidney Int, 66(2004):1589–1595.
[Crossref] [Google Scholar] [PubMed]
- B.E. Smid, S.L. Hoogendijk, F.A. Wijburg, C.E. Hollak, G.E. Linthorst, A revised home treatment algorithm for Fabry disease: Influence of antibody formation, Mol Genet Metab, 108(2013):132–137.
[Crossref] [Google Scholar] [PubMed]
- K. Nicholls, K. Bleasel, G. Becker, Severe infusion reactions to Fabry enzyme replacement therapy: Rechallenge after tracheostomy, JIMD Rep, 5(2012):109–112.
[Crossref] [Google Scholar] [PubMed]
- O. Aydin, C.S. Kasapkara, G.E. Celik, Successful desensitization with agalsidase alfa in 2 brothers with Fabry disease, J Investig Allergol Clin Immunol, 23(2013):367–368.
[Google Scholar] [PubMed]
- R.P. Limgala, J. Fikry, V. Veligatla, O. GokerâAlpan, The interaction of innate and adaptive immunity and stabilization of mast cell activation in management of infusion related reactions in patients with Fabry disease, Int J Mol Sci, 21(2020):7213.
[Crossref] [Google Scholar] [PubMed]
- I. DuBuske, K. Schmidlin, J.A. Bernstein, Successful desensitization of a patient with Fabry disease with agalsidase beta (Fabrazyme) anaphylaxis after omalizumab pretreatment, Ann Allergy Asthma Immunol, 126(2021):96.
[Crossref] [Google Scholar] [PubMed]
- J.E. Wraith, A. Tylki-Szymanska, N. Guffon, N. Guffon, Y.H. Lien, et al. Safety and efficacy of enzyme replacement therapy with agalsidase beta: An international, open-label study in pediatric patients with Fabry disease, J Pediatr, 152(2008):563–570.
[Crossref] [Google Scholar] [PubMed]
- A. Tanaka, T. Takeda, T. Hoshina, K. Fukai, T. Yamano, Enzyme replacement therapy in a patient with Fabry disease and the development of IgE antibodies against agalsidase beta but not agalsidase alpha, J Inherit Metab Dis, 33(2010):S249–S252.
[Crossref] [Google Scholar] [PubMed]
Copyright: © 2025 Lourdes de Fatima Ibanez Valdes, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.