Research: Journal of Drug and Alcohol Research (2022) Volume 11, Issue 10
Medication Reconciliation Led by Clinical Pharmacist as an Effective Strategy in Preventing and Reducing Adverse Drug Reactions
Yerni Kumar B1*, Chakravarthy Guntapalli2, Koteswar rao GSN3, Kishore Kumar K4 and Rajsekar A42Department of Pharmacognosy, Koneru Lakshmaiah Education Foundation, India
3Department of Pharmaceutics, Galgotias University, India
4Department of Pharmacology, Andhra University, India
Yerni Kumar B, Department of Pharmacy, Koneru Lakshmaiah Education Foundation, India, Email: chakra_varthy123@kluniversity.in
Received: 03-Oct-2022, Manuscript No. JDAR-22-80866;;Accepted Date: Oct 24, 2022; Editor assigned: 05-Oct-2022, Pre QC No. JDAR-22-80866 (PQ); Reviewed: 19-Oct-2022, QC No. JDAR-22-80866; Revised: 24-Oct-2022, Manuscript No. JDAR-22-80866 (R); Published: 31-Oct-2022, DOI: 10.4303/JDAR/236205
Abstract
Background and objectives: Alarming statistics of adverse drug reactions round the world is urging the health care professionals to focus on managing them in the best possible way. Although previous studies had focused on reporting the incidents and measured the severity of adr’s of most of the drugs. In contrast, our study is aimed to prove that implementing a few important strategies would prevent the adverse drug reactions and if occurred what efforts to be put in place in order to curb the incidence and severity. Preventing an adr would certainly imply the affirmation rendered to the patient by the clinical pharmacist and allied health care professionals.
Aim: The main purpose of this study is to assess the outcome of ADRs upon execution of medication reconciliation and robust training to the nurses in a tertiary care centre. Focus is on to prevent and reduce adverse drug reactions.
Methods: Medication reconciliation form was prepared in-order and approved the drugs and committee, started to document the past medical history of the patients, which also records the drug allergy status. The clinical pharmacist in collaboration with the nursing supervisors trained all the nursing staff based on the training calendar scheduled. Bed side and class room training attendance was recorded to ensure the training occurred. Evaluation is done after every training session. Going further, the practice of giving parenteral chlorpheniramine (anti-histamine) or hydrocortisone is initiated to prevent any allergic reactions of an antibiotic which is usually administered to the patient before starting the surgical procedure.
Results: It was found that, of 410 patients observed in this study, 9 adverse drug reactions were evidenced ranging from mild to moderate and rest of the patients had no reactions. Statistical analyses tool was used to assess the data. Among the 12 adrs occurred it is antibiotics and snake venom that caused most of the adrs 4 adrs were seen in the patients admitted in general medicine department, 3 in the department of orthopaedics, 1 in critical care and anesthesia department, also 1 in pulmonology. The total adr figure has dropped from 17 to 9 post training, evaluation, post start of administering single shot of an antihistamine or hydrocortisone as well, implementation of the other strategies. 17 adverse drug reactions were reported before the implementation of the above strategies discussed. As a routine review and follow up, these adr’s were observed. As “human life is priceless” even a single adr must be considered serious as it can potentially affect the patient. The credit goes to the introduction of previous medication history collecting form as well consistent practice of giving a test dose is another key factor which contributed to insignificant and less severe adr’s recorded.
Conclusion: Medication reconciliation and In patient medication review done by clinical pharmacists, training offered by them to the nurses at all levels and administering a single dose of an antihistamine before the surgical procedure has shown remarkable decline in the outcome of number of adrs as well their intensity also. Preventing an adverse drug reaction in the hospital setting which means improving the quality of life of the patients.
Keywords
Antibiotics; Adverse drug reactions; Clinical pharmacist; Vasopressors; Hypotension, other drugs
Abbreviation
(ADR) Adverse Drug Reactions; (CP) Clinical Pharmacist; (VP) Vasopressors
Introduction
Millions of patients around the world are afflicted with adverse drug reactions every year. An ADR is defined as a noxious, unintended event that affects the patient significantly or insignificantly [1,2]. ADRs are broadly classified into predictable and unpredictable type. Adverse drug reactions will range from mild to severe depending on damage they cause to the patient. Mild adr’s doesn’t affect the patient and they do not require much attention as they resolve themselves with little care. Moderate adr’s can be healed by stopping the drug to which the patient is allergic. Severe adr’s do have the potential to cause significant harm to the patient [3,4]. These require precise interventions to find the root cause and based on the root cause found, a perfect analysis must be done. This way a few adr’s can be prevented in future form happening. One of the prominent strategies to prevent an adr is certainly doing flawless medication reconciliation.
Drugs are chemical compounds that alleviate human suffering from many ailments. Almost every drug in pharmacology will be producing some or the other side effects. From a basic oral ulcer to an excruciating neoplasm, uses of drugs are inevitable and with the use of them side effects are also common that ranges from mild to severe. As the famous saying goes “Drugs are Double Edge Swords”, which means on one hand, they heal the disease, on the other hand they may act as toxin if used inappropriately, so it is important to use the drugs with great care [5]. Adverse drug reactions are untoward events that are painful. More than million patients around the world are hitting with adr’s on daily. The unfortunate thing with severe adr is its devastating side effects. The series of events vary from a small rash to severe bradycardia. Upon exposure of subsequent allergen that could be a chemical or an external substance, binding of IgE antibodies takes place on the mast cell. Mast cell degranulation causes the release of histamine into the blood circulation. Upon its release, histamine produces three physiological actions. Firstly, histamine vasodilates the blood vessels which as a result causes hypotension-steady fall in blood pressure. Followed by vasodilatation, due to increase in the capillary permeability, accumulation of plasma takes place that causes oedema, redness as a result of blood pooling, eventually pain arises as a consequence of stimulating the peripheral nerve fibres [5,6]. Reduced blood pressure may decrease the heart rate that lands up in bradycardia. With all the above abnormal events, there is a chance that patient may end up in systemic shock [6].
Anaphylaxis is a systemic phenomenon projected in the form of severe allergic reactions. Following intense allergic reactions a cascade of events takes place and to alleviate this situation, vasopressors must be initiated with an appropriate dose. Commonly used vasopressors include Dopamine and Nor-adrenaline. Once the blood pressure returns to normal the vasopressors must be stopped gradually but not abruptly to avoid sudden fall in BP. To reduce the inflammation on the skin, corticosteroids must be initiated and to be continued until the inflammation subsides [7].
Materials and Methods
This cross sectional study was conducted in a tertiary care hospital at Andhra Pradesh, Southeast India for a period of 10 months. Patients above 25 years who received one or more medication for the disease condition were enrolled in this study and patients of below 25 years were excluded. Medication reconciliation form was prepared in-order to document the past medical history of the patients, which also records the drug allergy status [7,8]. This form was prepared by a team of health care professionals which was approved by the drugs and therapeutic committee. It is the onus of a clinical pharmacist to have a structured medication review with the patient and as a part of this, all previous medications which the patient is using must be noted in that form, eventually will be reviewed by the treating physician. Based on the clinical needs of the patient the physician will decide which medications to be continued and which to be discontinued. The whole process often takes place in the emergency ward within a few minutes after the patient gets admitted. Once the form is filled completely, one copy will be placed within the file of the patient, the other within the record of clinical pharmacology department. With the comprehensive medication review, the cp will be able to know previous history of drug allergy status [8]. The antibiotic consumption data is collected through online tracking, a passive data collection that traces information in digital environment and the medication reconciliation forms however are conventional type in collecting the data.
Upon finalizing the medicines to be continued, they must be entered to the medication chart by the duty medical officer and these medication orders will be stringently abiding by the concerned nurse. During medication reconciliation, it is imperative that the clinical pharmacist asks about history of drug allergy status and jots down in the reconciliation form. With this, the work of cp is not close to over. Of the existing drugs in pharmacology, it is the antibiotics which have an extreme potential to cause adverse drug reactions. The C.P must ensure that patient does not receive any previous allergic drugs or any drugs of the same pharmacological classification. Not only that, it must be ensured that before initiating any antibiotic, especially the high end antibiotics which have allergic tendency must be administered with 0.1 ml intradermally. Followed by that, observe for the allergic manifestations such as rash, itching and swelling in the patient [9,10]. The gold standard practice includes a precautionary measure to run the antibiotic slowly through an intravenous route, so that the adr can be noticed when it is at mild level. With the above strategy instant measures can be taken to reduce the severity and incidence of adverse drug reactions.
On a week or bi-weekly the nursing staff are taught and trained on giving a test done before the actual administration of the drug, that could be an antibiotic and any other drug which has a potential to cause significant adverse drug reaction. Physicians, nurses and pharmacists have done this study in collaboration with each other. The clinical pharmacist in collaboration with the nursing supervisors trained all the nursing staff based on the training calendar scheduled [11,12]. They were taught to administer a test dose for high risk medications and antibiotics before the actual infusion begins. The staffs were assessed on stuff learned during the training. Bed side and class room training attendance was recorded to ensure the training took place. Apart from this, they were also trained to escalate to the primary doctor and clinical pharmacist, if there is any evidence of hypersensitive reactions observed for any drug they give to the patient. The reason behind this is, early intervention and treatment can reduce the intensity of adr and its detrimental effects. On the other hand, the practice of giving parenteral chlorpheniramine or hydrocortisone is initiated to prevent any allergic reactions from the antibiotic which is usually administered to the patient before starting the surgical procedure and shifting the patient to operation theatre [12].
Results
The outcome of this study was proved to be productive which is immensely beneficial to the patient thus alleviating the patient care. The number of adverse drug reactions significantly dropped post training and evaluation, which highlights the importance of strategically training and its implementation. Percentage analysis of the collected data was performed. It was found that, of 410 patients observed in this study, 9 adverse drug reactions were evidenced ranging from mild to moderate and rest of the patients had no reactions. Statistical analyses tool was used to assess the data. Among the 12 adrs occurred it is antibiotics and snake venom that caused most of the adrs. 4 adrs were seen in the patients admitted in general medicine department, 3 in the department of orthopaedics, 1 in critical care and anesthesia department, also 1 in pulmonology. The total adr figure has dropped from 17 to 9 post training, evaluation and implementation of the other strategies.
17 adverse drug reactions were reported before the training took place. As a routine review and follow up, these adr’s were observed. As “human life is priceless” even a single adr must be considered serious as it can potentially affect the patient. The credit goes to the introduction of previous medication history collecting form as well the consistent practice of giving a test dose is another key factor which contributed to less intense and less severe adr’s. Also, there were no adr’s seen so far, after starting the practice of administering of parenteral antihistamines for the patients who receive an antibiotic undergoing surgery (Tables 1 and 2) (Figure 1).
| Department | Pre training | Post training |
|---|---|---|
| General medicine | 5 | 4 |
| Critical care | 2 | 1 |
| Orthopaedics | 6 | 3 |
| Pulmonology | 3 | 1 |
| Others | 1 | 0 |
| Total | 17 | 9 |
Table 1: Comparative results of pre and post training about the preventing and handling of an ADR
| Department | n (%) | |
|---|---|---|
| Pre intervention (n=17) | Post intervention* (n=9) | |
| General medicine | 5 (1.2) | 4 (0.9) |
| Critical care | 2 (0.4) | 1 (0.24) |
| Orthopaedics | 6 (1.4) | 3 (0.73) |
| Pulmonology | 3 (0.7) | 1 (0.24) |
| Others | 1 (0.24) | 0 |
Table 2: Percentage of adverse drug reactions in each department before and after clinical pharmacist intervention among inpatients
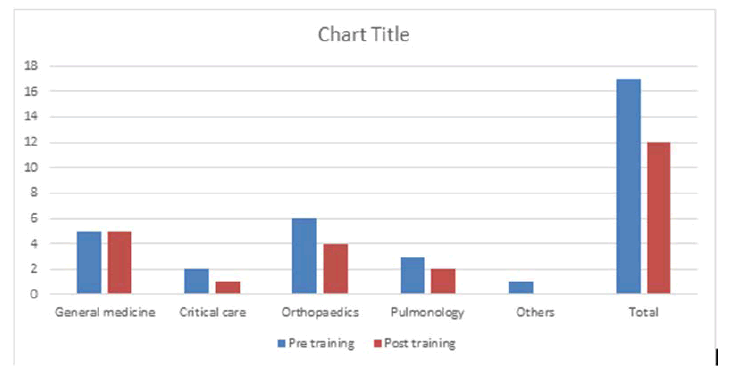
Figure 1: Illustration of number of adr’s in each department pre and post training on preventing the ADR
Pre-intervention ADRs among various departments include 1.2% in general medicine, 0.4% in critical care, 1.4% in orthopedics and 0.7% in pulmonology. Post-intervention ADRs varied in different departments. Most predominantly adrs were found in the department of general medicine 4 (0.9%) and the probable reason for this is, most of the clinical cases fall under this department unless referred to a superspecilaity. The next high number of adverse drug reactions occurred in orthopedics 3 (0.73%) followed by that which took place in the critical care 1 (0.24%). A lack of intervention by a clinical pharmacist and partially equipped safe infusion practices is known to be the common cause of ADRs and their severity. In most of the ADRs, we haven’t rechallanged the same drug to the patient as we are certain that hypersensitivity reactions such as urticaria, rash and itching are produced by the drugs administered.
Discussion
One must remember that it is not only antibiotics, every drug in the world of pharmacology has a potential to cause side effects. There is no wonder in saying that there isn’t any drug exists devoid of side effects. Here we describe one incident where a patient has ended up with Anaphylaxis which is really painful, where test dose of Ceftriaxone was not administered. In general, if a patient is having any co-morbidity and has to be taken up f or surgery, the concerned surgeon will give referral to the specialist to evaluate and opine the patient status. The surgeon takes further step based on the opinion given by the physician. If the patient falls into standard and mild risk category as per the opinion of the specialist, then the surgeon decides to operate the patient [13]. The patient was kept on NBM from the previous day night as the surgery was planned in the wee hours of next day. Before shifting patient to operation theatre the next day, an antibiotic is given as a part of preoperative prophylaxis.
Within a few minutes before shifting to operation theatre, patient started to feel chills, cutaneous rash and flush. Followed by, the extremities and facial region got swollen. Going further, the patient blood pressure dropped which is probably due to the action of histamine on peripheral blood vessels causing hypotension which is associated with tachycardia. In these extra crippling circumstances, vasopressors were swiftly initiated to counteract the fall in blood pressure. Vasopressin and dopamine intravenous infusions were immediately started at a controlled rate until the blood pressure returned to normal. Fortunately, within an hour the vitals gradually started to become stable and patient got recovered, eventually discharged on the next day in a hemodynamically stable state. Therefore, it is understood antibiotic sensitivity testing is paramount before administering an antibiotic which can prevent any untoward consequences. Most importantly histamine and other inflammatory mediators are thought to blame for the whole vicious cycle. It’s a deeply rooted misconception that allergic manifestations such as rash, pruritis, itch and swelling shall only fall under adverse drug reactions. There are still many types of adverse effects which include dose related, idiosyncratic, teratogenic and phototoxic effects (Table 3).
| Drug | Most commonly observed Adverse effects | Probable mechanism involved |
|---|---|---|
| Glyceryl nitrate | Dizziness , Headache | Vasodilatation mediated hypotension |
| Canagliflozin | UTI | Re absorption of glucose from the renal tubule |
| Warfarin | Elevated INR subsequent bleeding | Antagonising the action of vit K |
| Parenteral corticosteroids | Hyperglycemia | Rapid glucocorticoid metabolism |
| Diuretics | Electrolyte imbalance | Inhibition of sodium and potassium ions |
| NSAID’s | Acute gastritis | Blocking the mucoprotective role of COX |
| Long acting sulfonamides | Hypoglycaemia | Increased plasma half life |
| Pregabalin | Intense sedation | Aggressive enhancement of GABA |
| Isoniazid | Pins and needles sensation, burning foot | Pyridoxine deficiency |
| Morphine | Constipation | Blocking the Ach release from the nerve endings. |
Table 3: The most common drug and its dose related adverse effects that are clinically observed in this study include
Almost every drug in world of medicine has a potential to cause an adr, however the above are examples of a few drugs which caused adr even at the therapeutic doses when used for a week to month duration. Dose-related ADRs are usually predictable. ADRs unrelated to dose are usually unpredictable. Dose related side effects are commonly observed when a patient is treated with an aggressive dose of the drug. Drugs with narrow therapeutic index are the ones with small differences between therapeutic and toxic doses [14,15]. A higher dose of any medication, especially the narrow therapeutic drugs with a small increase in their dose cause immediate adverse effects. This is the reason the plasma concentration of these drugs must be monitored closely. Upon any increase of the drug concentration in plasma beyond therapeutic levels, the dose must be titrated accordingly by the physician to avoid toxic effects of the drug. The process by which the plasma levels of these drugs are measured is called therapeutic drug monitoring. On a whole, it is clear that plasma concentration of narrow therapeutic drugs must be monitored to ensure patient safety. Narrow therapeutic drugs include aminoglycosides, ciclosporin, carbamazepine, digoxin, lithium, phenytoin, phenobarbital, rifampicin, theophylline and warfarin. Monitoring the effects of drugs either by measuring their direct serum concentration or by measurement of physiological markers is one strategy to reduce the severity of adr. Clozapine, used for the management of resistant schizophrenia or psychosis is associated with risk of agranulocytosis [15].
Periodic monitoring of white blood cell count has effectively eliminated the risk of fatal aggranulocytes. Even from the manufacturer’s side, it is their onus to mention the risk in a caution box on the drug label. Monitoring requirement must be labeled on the packaging strip. Drug information centres must be setup as they will be providing reliable information to the customers about the monitoring requirements of the drug the patients use. Patients must increasingly have an access to the information of drug induced adr and this can be done through contacting health care professionals and drug information centers. They must be taught on how to manage the consequences of adr as moderate to severe adr effects are something that can’t wait until the patient arrives to the casuality [15,16]. One easy way of communicating about the adr to the general public is displaying the cartoons which would easily be understood by them. Perhaps, physicians has right to prescribe, but after knowing the information about the medicine and its untoward effects upon over dose, it is final decision of the patient to decide whether to continue or not.
The article also discuss how important is pharmacovigilance in re-discovering the new pharmacological properties of marketed drugs eventually making people’s life better. With regard to the above, a few examples were discussed to prove that pharmacovigilance made a strong impact in the world of pharmacology. Pharmacovigilance otherwise known as Post marketing surveillance study which is classified as phase IV clinical trial. Pharmacovigilance and adverse drug reactions are closely related to each other. A few adverse effects which are not seen in the first three phases of the clinical trials can be observed in the phase IV. The reason for this is, a drug once released into the market after getting a nod from food and drug administration will be consumed by numerous patients, of course on the prescription of a registered medical practioner. The same medicine will be consumed by healthy volunteers and patients during the clinical trials, number of participants in these trials will be limited, and as a result the chance of adr occurrence can be mere [17]. The true characteristics of any drug shall be seen only when it is taken by a large number of patients.
It must be understood that adverse effects in a few circumstances can be turned out into therapeutic uses. One such drug is minoxidil, where its adverse effect was utilised as a therapeutic effect in the treatment of male pattern baldness. Minoxidil is basically an antihypertensive which produces its vasodilatory action mediated by opening of potassium sensitive ATP channels. On the other hand, it promotes hair growth by improving the viability of hair follicles and this physiological effect was discovered when the drug is used extensively. The unwanted hair growth was noticed because of the monitoring as a part of pharmacovigilance.
Metoclorpramide is primarily an anti-emetic which works by antagonizing the dopamine (D2) receptors in the chemoreceptor trigger zone. It also acts by increasing the secretions of acetylcholine thereby enhancing peristaltic movement of the stomach. The above actions enhance the movement of food from the stomach into intestine emptying the contents of stomach which are considered is the routine pharmacological actions. Metoclorpramide has a special value in obstetrics and gynaecology. It is used to eject the milk from mammary glands and this pharmacological effect is observed during the post marketing surveillance studies on insufficient lactating mothers. The laboratory analyses were performed after 15 days of the drug therapy both in control and experimental groups. Daily milk production increased by 28.5% which was statistically significant. The mechanism by which metoclorpramide promotes lactation is antagonizing the release of dopamine in the central nervous system, thereby increasing prolactin levels, eventually augmenting breast milk levels [17,18].
Codeine, another remarkable drug that was originally used to suppress severe pain. Supraspinal and cancereous pain are usually controlled with this drug. Codeine binds to the opioid receptor and produces agonist like action which is similar to endorphins and enkephalins produced naturally in the brain. Codeine depresses the cough reflex, partly by a direct effect on a cough centre in the medulla while the exact mechanism is not entirely clear. Suppressing the incoming reflexes stops the medulla from being activated thus active cough isn’t produced. However, in the recent years, codeine containing preparations, especially the syrup forms are banned from being sold because of its extreme addiction potential. Consuming high doses of codeine has the potential to produce respiratory paralysis which leads to cessation of lung function. It is clearly evident that drugs are double edged swords and they must be used with caution and a rationale.
Adverse drug reaction also includes teratogenicity which is a major concern for pregnant women. Any drug which is having a capacity to cause foetal abnormalities is considered to be teratogenic. Drugs during pregnancy must be used with great caution to avoid any complications to the mother as well the foetus inside. Self-medication is dangerous, so use of drugs during any one of the three trimesters must be on the advice of a registered medical practioner only. Inappropriate conclusion of the clinical trial data on the new drugs can misguide the physician to prescribe them eventually leading to detrimental consequences. One incident that had left many mothers in tears is the “Thalidomide Tragedy” which shook the whole world [19].
It’s a common phenomenon for vomiting to occur during the first trimester of pregnancy and to prevent this; antiemetic named thalidomide was widely prescribed. There is no deny that thalidomide is a potent one and was once considered as drug of choice for the prevention of emesis. All the pregnant mothers who swallowed this drug got an immediate relief from vomiting and seemed to be comfortable. Here comes the unpredictable outcome after taking this drug often in the pregnancy time’s i.e the neonates of the mothers were born with sealed limbs which is referred to phocomelia. This incident came into limelight after noticing that most of the mothers who used thalidomide delivered babies with absolute limb deformity. All the mothers eventually were in deep agony after looking at their new born babies. The prominent reason for this devastating incident to occur is the health care or medical fraternity did not consider the testing of drug on pregnant animals during pre-clinical studies. Surpassing the conduction of pre-clinical studies of thalidomide, there weren’t any results produced. However, the existing data of thalidomide pertinent to control of nausea and vomiting was adequate to prescribe it, eventually it was prescribed. As per few reports round the world, the victims of the thalidomide tragedy were still fighting or demanding for justice in terms of huge compensation. With the above incident, the regulations have become stringent to conduct the drug trails on volunteers. The government of every nation made it mandatory to conduct at least animal studies to know whether the experimental drugs are having teratogenic potential [19,20]. The most prominent of all is to project the data in a transparent manner.
Majority of the ADRs are thought to be preventable, hence it is important to dramatically reduce the expenses associated with ADR. Recent studies indicate that between 55% to 73% hospital admissions in the elderly are likely to be preventable. ADRs can be prevented by checking the previous ADR history, decreasing the use of drugs that carry high potential of adr and considering the factors predisposing to adr while prescribing. Sharing information about adrs that occurred in the medical institutions and hospitals is vital. The adrs must be reported to the nearest adverse monitoring centre or the pharmacovigilance society of the concerned nations. Clinical Pharmacists have a social and professional responsibility to perform one to one medication reviews especially for the patients who are on complex polypharmacy. A latest study evidenced medication review’s performed by cp is immensely helpful in stopping the unnecessary drugs which are no longer beneficial to the patients. To do this, the cp must discuss with the physician before modifying the prescription. Modification includes stopping or changing the medication orders prescribed. The C.Ps are empowered to practice to the best of their ability in developed countries. Well, alot more has to be done to enhance the credibility of the profession in the developing countries. Management of medication is a huge chapter in the hospital setting which is a big responbility on the shoulders of a clinical pharmacist. They are certainly medication experts and are expected to answer the medication queries raised by any health professional.
Irrational prescribing is another component that can contribute to adr. Often patients with multiple co-morbidities will have to visit more than one clinician and here arises the problem. Without the knowledge of one, the other clinician might prescribe medicines of the same pharmacological category or same composition that of different brand. Keeping the health condition in their mind, patients start taking all the medicines advised by their physician which is not only unnecessary, but have the potential to harm. Harmful effects of drugs may be expressed as an adverse drug reaction by the human body, because administering any drug beyond their therapeutic dose causes toxic effects which we usually call it as adr.
Communication is a key factor for the prevention of adr. It is important to have a precise communication between any two health care professionals may be a nurse and a doctor or a clinical pharmacist. Fatalities related to adr can be decreased if the prior allergic history is known to any health care provider who handles the patient. The patient attenders must voluntarily render information to the health care professional who seeks. If this is not possible, the physician or cp must question the patient for any uncomfortable feeling or hypersensitivity upon administration of medicines. In most of the situations communication is a barrier, so the target must be to overcome it. Communication can be improved by having a translator at the work place. Patient arriving to the treatment centers may not be well versed with the local language which would certainly be a barrier for them to communicate. Here comes the crucial role of a translator who acts as a mediator between the patient and a physician or any other health care professional. Ideally, a translator must be having a strong command on more than three to four languages of the neighbouring states and obviously national language. Drug Induced renal toxicity as an adverse drug reaction.
Our study had a special emphasis on drug induced renal toxicity, as kidney is a major organ for detoxification of most of the drugs. Drugs that have a nephrotoxic potential upon chronic use can damage the internal texture and cause glomerular nephropathy, tubular damage and interstitial nephritis. Progressive renal failure is characterized by a gradual increase in serum creatinine levels that may result from loss of function of sufficient nephrons. Patients above 40 years with arthritic pain take non-steroidal anti-inflammatory drugs rampantly. With nsaids, mild to moderate pain may subside and relieve the pain symptomatically but its long term consequences are debilitating. With the use of such over the counter drugs such as analgesics for more than weeks, there is every chance that patients will end up in acute kidney injury. Of 53 patients, 29 of them were observed that nsaids are the most common agents causing acute renal injury. The next category of drugs causing renal failure is angiotensin converting enzyme inhibitors. There is no denying that Ace inhibitors are the drug of choice in delaying the progression of hypertensive complications such as diabetic nephropathy and neuropathy, thus decreasing the excretion of proteins in the urine [20]. Patients who were diagnosed with acute or chronic kidney disease were seen prescribing telmisartan or enalapril with a view to delay the onset of diabetic nephropathy. But these drugs do more harm than benefit in patients with nephropathy which manifests as increase in serum creatinine. Thus it is the stage where the ace inhibitors or arbs must be stopped.
Amino glycosides such as amikacin and gentamicin are widely used in the clinical practice. Amikacin is generally used for the treatment of uncomplicated to complicated urinary tract infections as well, post-operative urinary tract infections. These drugs are found to inhibit lysosomal hydrolases in the proximal tubules of the kidney which manifests in the form of acute tubular necrosis. Another drug amphotericin B for the treatment of susceptible fungal infections is also having the potential to cause nephrotoxicity. Amphotericin induced renal injury appears to occur via a similar mechanism with initial binding of this polyene antibiotic to membranes of renal tubular epithelial cells. Because the mechanism responsible for its action is similar to mechanism responsible for toxicity, the margin between dose of the drug required for antifungal activity and renal injury is very small. So any small increase in the dose of this drug leads to a high frequency of renal injury in patients receiving amphotericin B [21,22]. However liposomal formulations of amphotericin were developed to reduce the toxicity as well, to increase the plasma half-life of the drug which is certainly a beneficial effect.
Conclusion
The study witnessed the immense benefit of implementing strategies such as giving a test dose before the actual administration of an antibiotic infusion and collecting the previous medication history by a clinical pharmacist. Also, ensuring that the known allergic drug or any drug of the same pharmacological classification is not prescribed as well not administered by performing an intense medication review. Also, Administration of single dose of an antihistamine before the surgical procedure. Undoubtedly, clinical pharmacists are vital members of the health care team because every small contribution they make would definitely bring a difference in the quality of life of the patients. In a nutshell, it is important that every tertiary health centre would adopt a competitive clinical pharmacist to improve their patient care services and reduce the burden on medical fraternity.
Acknowledgement
None.
Conflict Of Interest
Authors have no conflict of interest to declare.
References
- M.M. Elamin, K.O. Ahmed, O.K. Saeed, M.A. Yousif, Impact of clinical pharmacist-led medication reconciliation on therapeutic process, Saudi J Health Sci, 10(2021):73.
- R.A. Tariq, R. Vashisht, A. Sinha, Y. Scherbak, Medication dispensing errors and prevention. StatPearls: Treasure Island, USA, 2020.
[Google Scholar] [PubMed]
- National Patient Safety Goals
- Medication Reconciliation Review | IHI-Institute for Healthcare Improvement, (2015).
- K.P Lee, C. Hartridge, K. Corbett, E. Vittinghoff, A.D. Auerbach, “Whose job is it, really?” physicians, nurses, and pharmacists perspectives on completing inpatient medication reconciliation, J Hosp Med, 10(2015):184–186.
- N. Chamoun, U. Usta, L.R. Karaoui, P. Salameh, S. Hallit, et al. Current trends in hospital pharmacy practice in Lebanon, Hosp Pharm, 55(2020), 112-118
- Ghusn H, Polypharmacy: What clinicians need to know while caring for an elder, J Med Liban, 60(2013), 207–213.
[Google Scholar] [PubMed]
- E. Ramia, R.M. Zeenny, S. Hallit, P. Salameh, Assessment of patients’ knowledge and practices regarding their medication use and risks in Lebanon, Int J Clin Pharm, 39(2017), 1084–1094.
- K.S. Boockvar, S.L. Santos, A. Kushniruk, C. Johnson, J.R. Nebeker, Medication reconciliation: Barriers and facilitators from the perspectives of resident physicians and pharmacists, J Hosp Med, 6(2011), 329–337.
[Crossref] [Google Scholar] [PubMed]
- M.M. Geurts, J. Talsma, J.R. Brouwers, J.J. de Gier, Medication review and reconciliation with cooperation between pharmacist and general practitioner and the benefit for the patient: A systematic review, Br J Clin Pharmacol, 74(2012), 16–33
- Pal A, Babbott S, Wilkinson ST, Can the targeted use of a discharge pharmacist significantly decrease 30-day readmissions? Hosp Pharm, 48(2013), 380–388.
- A.H. Salanitro, C.Y. Osborn, J.L. Schnipper, C.L. Roumie, S. Labonville, et al. Effect of patient-and medication-related factors on inpatient medication reconciliation errors. J Gen Intern Med, 27(2012), 924–932
- W.A. Tumwikirize, J.W. Ogwal-Okeng, A. Vernby, W.W. Anokbonggo, L.L. Gustafsson, et al. Adverse drug reactions in patients admitted on internal medicine wards in district and regional hospitals in Uganda, Afr Health Sci,11(2011), 72–78.
[Google Scholar] [PubMed]
- D.I. Rappaport, B. Collins, A. Koster, A. Mercado, J. Greenspan, et al. Implementing medication reconciliation in outpatient pediatrics. Pediatrics, 128(2011), e1600–1607
- Australian Commission on Safety and Quality in Healthcare (2016) Taking a Best Possible Medication History.
- S. Mueller, K.C. Sponsler, S. Kripalani, J.L. Schnipper, Hospital-based medication reconciliation practices: A systematic review, Arch Intern Med, 172(2012), 1057-1069.
- Boockvar KS, Santos SL, A. Kushniruk, C. Johnson, J.R. Nebeker, Medication reconciliation: Barriers and facilitators from the perspectives of resident physicians and pharmacists, J Hosp Med, 6(2011), 329-337
- A. Bennett, D. Gnjidic, M. Gillett, P. Carroll, S. Matthews, et al. Prevalence and impact of fall-risk-increasing drugs, polypharmacy, and drug–drug interactions in robust versus frail hospitalized falls patients: A prospective cohort study, Drugs & Aging, 31(2014), 225-232.
- X. Feng, X. Tan, B. Riley, T. Zheng, T. Bias, et al. Prevalence and geographic variations of polypharmacy among West Virginia Medicaid beneficiaries, Ann Pharmacother, 51(2017), 981-989.
- K.L. Flood, M.B. Carroll, C.V. Le, C.J. Brown, Polypharmacy in hospitalized older adult cancer patients: Experience from a prospective, observational study of an oncology-acute care for elders unit. Am J Geriatr Pharmacother, 7(2009), 151-158.
- M. Greene, M. Steinman, I.R. McNicholl, V. Valcour, Polypharmacy, drug–drug interactions, and potentially inappropriate medications in older adults with human immunodeficiency virus infection. J Am Geriatr Soc. 62(2014), 447-453
- A. Bandura, Self-efficacy: The Exercise of Control, W.H. Freeman and Company, New York
Copyright: © 2022 Yerni Kumar. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.

