Research: Journal of Drug and Alcohol Research (2022) Volume 11, Issue 10
Levels of Polycyclic Aromatic Hydrocarbon and Polychlorinated Biphenyl in the Mainstream Smoke of Cannabis Sativa and Health Implications
Olusola Adedayo Adesina1*, Olowolafe Tosin1, Mayowa Lala2, Abolaji T. Adeyemo2 and Jacob Sonibare32Department of Medical Microbiology and Parasitology, Uniosun Teaching Hospital, Nigeria
3Department of Chemical Engineering, Obafemi Awolowo University, Nigeria
Olusola Adedayo Adesina, Department of Chemical and Petroleum Engineering, Afe Babalola University, Nigeria, Email: adesinaolusola50@yahoo.com
Received: 03-Oct-2022, Manuscript No. JDAR-22-78742;;Accepted Date: Oct 24, 2022; Editor assigned: 05-Oct-2022, Pre QC No. JDAR-22-78742 (PQ); Reviewed: 19-Oct-2022, QC No. JDAR-22-78742; Revised: 24-Oct-2022, Manuscript No. JDAR-22-78742 (R); Published: 31-Oct-2022, DOI: 10.4303/JDAR/236203
Abstract
This study determined the levels of Polycyclic Aromatic Hydrocarbon (PAHs) and Polychlorinated Biphenyls (PCBs) in the mainstream smoke of some samples of marijuana. The study also assessed the risk associated with smoking PAHs and PCBs in the marijuana using health indexes such as incremental lifetime cancer risk (ILCR) and hazard quotient (HQ). Samples of marijuana were obtained and analysis of PAHs and PCBs were done using GC-MS operated in selected ion monitoring mode (SIM). The result showed the mean Σ PCBs in the smoke was 25.86 ng while the concentration of Σ dl-PCBs was 11.04 ng. `The mean Σ PAHs in the smoke was 1363.61 ng. The ILCR values from smoking PCBs in the mainstream ranged between 6.98 × 10-10-1.38 × 10-9 while the ILCR value for exposure to PAHs was 1.75 × 10-5. HQs values for exposure to PAHs and PCBs in smoke were all above 1. The result showed no carcinogenic risk is associated with exposure to PCBs in the mainstream of marijuana, while both carcinogenic risk and non-carcinogenic risk are associated with exposure to PAH in the smoke. The study revealed risks associated with smoking marijuana.
Keywords
Cancer risk; Hazard quotient; Mainstream smoke; Marijuana
Introduction
Marijuana is one of the most used illicit substances worldwide in 2016, about 3.9% of the total world population aged 15-64 making up 192.2 million people used Marijuana (World Drug Report, 2018) [1]. Global deaths caused by the use of these illicit drugs increased by 60% in 2018 [2]. Marijuana is often perceived as a natural product, as it is a flowering plant with a unique aroma, despite its illegality in most countries, it is used by many for its potential medicinal purposes [3,4]. Some states in the U.S have legalized the use of some amount of Cannabis, this called medical marijuana. However, smoking this product is considered by many as less harmful than tobacco smoking [1,2,5]. Nevertheless, smoking involves combustion, and Marijuana smoke may contain some carcinogens and many other harmful substances usually emitted during the smoking process [5]. The most common method of smoking marijuana is the inhalation of the smoke of the dry plant of the cannabis flower rolled up in a thin paper similar to cigarettes and cigars [6,7], other methods of marijuana smoking include the use of acrylic water pipe and a vaping pen that vaporizes cannabis liquid [7-9]. The amount of smoke produced by the different smoking methods differs from one another; the smoking of the dry plant in a thin paper roll produces more particulate matter than the other methods such as water pipe and concentrates hash [10]. Unlike tobacco and cigarette smoking, Marijuana is smoked without filters and has smaller butt sizes, which leads to the inhalation of higher smoke concentration [11].
Polycyclic Aromatic Hydrocarbons (PAHs) are a class of pollutants that are persistent in the environment. The incomplete combustion of substances is a primary source of PAHs [12]. A smoker is exposed to several of these compounds with each puff of a lit tobacco or marijuana product [13].
Polychlorinated biphenyls (PCBs) are a part of persistent organic pollutants; they are synthetic chemicals that do not occur naturally in the environment (WHO Regional Office for Europe, 2000) [14]. They can also be produced by the combustion of compounds containing chlorine. It is worthy of note that various papers used as cannabis rollers are bleached paper with chemicals containing chlorine and other compounds. Another advantage of these papers is that it makes the cannabis burn slowly when smoking. However, combustion of these bleached papers with cannabis could likely lead to the formation of PCBs.
Although some countries have embraced the legalization of Marijuana for medical use, most countries especially in Africa are yet to legalize the substance use. There is less extensive research on the chemical composition of marijuana mainstream smoke consumed in most African countries like Nigeria. Despite the government ban on these products, the use of cannabis and other drugs in Nigeria continues to rise [15].
Most studies on Marijuana in Nigeria focus on the role of individual-level factors, peer influence, the influence of family/household-level, normalization, motivation, and social factors [2,15-19], only a few studies have been carried out on the chemical constituent and the pollutants present in the marijuana smoked in Sub-sahara countries of Africa, such Nigeria. This study investigates the concentration of Polycyclic Aromatic Hydrocarbons (PAHs) and Polycyclic Biphenyls (PCBs) present in the samples of marijuana smoke acquired in the south-western part of Nigeria. The study also calculated the risk associated with marijuana smoking using some health indexes.
Materials and Method
Different marijuana samples were obtained in Ado-Ekiti, Western Nigeria. An improvised smoking machine as described by Genller, et al. was used to collect mainstream smoke [20]. This apparatus has also been used by Adesina et al. for the collection mainstream of cigarettes [21]. The apparatus consists of glass bottles, a glass syringe, a conical tube cap, and two polypropylene tubing. Dichloromethane (DCM) was used as a solvent in the smoking machine apparatus. The experiment was carried out in a fume cupboard, the marijuana stick was lit, with the aid of the syringe, a 35 ml puff was drawn into the solvent, lasting 2 seconds-4 seconds, and this was repeated every 30 seconds until the marijuana roll was exhausted. The PAHs and PCBs are extracted into the DCM, the extract was properly stored and labeled them sent for immediate analysis.
Clean-up procedure
The clean-up procedure was done with the use of 5 g of silica gel column, eluted with 40 mL 1:1, DCM: Hexane [22]. 20 ml of the extract was concentrated using a rotary evaporator under a gentle stream of nitrogen. PAHs and PCBs quantification in the sample was carried out using a gas chromatography-mass spectrophotometer (GC-MS). The GC (Agilent 7890) with a mass detector (Agilent 5975) operated in selected ion monitoring mode using electron impact ionization. With a chromatographic column dimension of 30 m × 0.25 mm and an internal diameter of 0.25 μm film thickness.
Sample analysis for PAHs 1 μL extracted sample was injected in splitless mode using helium as a carrier gas at a constant flow of 1.2 mL min−1. The temperature program for the analysis was set as follows: the initial temperature (50°C) was held for 2 minutes, ramped raised to 120°C at 30°C min−1, and then ramped again to 280°C at 6°C min−1 for 15 minutes [22]. The internal calibration method was used for the quantification of PAHs concentration in the samples. Isotopically labeled internal standards of PAHs (naphthalene-d8 and benzo(a) anthracene-d12) in dichloromethane were used for calibration. PAHs were identified and quantified based on their retention times, target qualifier ions, and internal standard calibration.
Sample analysis for PCBs
1 μL volume of sample injected into the GC-MS in splitless mode with initial temperature was placed at 120°C with 1 minute holding time, increased to 190°C at 20°C min-1, further increased to 230°C at 5°C min-1 and final temperature at 300°C at a rate of 25°C min-1 (10 minutes holding time). Injector and transfer line temperatures were set at 280°C and 300°C respectively. The carrier gas used was high purity Helium with a constant flow rate of 0.8 ml min-1. The sample extracts were spiked with 100 ng of PCB standard as the internal standard. The resulting solutions were concentrated into 100 μL and transferred into a 200 μL glass insert contained with a 2 mL amber autosampler vial for samples. All the PCB congeners, in the chromatographic peaks, were analyzed (Hu, et al., 2011).
Quality assurance/quality control (QA/QC)
A procedural blank was analyzed along the smoke samples to monitor any interference and contamination. The blank was made by pouring 5 ml of DCM into the extractor and was left for 3 minutes; it was then analyzed for PAHs and PCBs. Also, Analysis of samples was done in duplicates.
Health implications
Toxicity equivalence: The potential toxicity of the PCBs was calculated using the toxicity equivalence factor. The values are calculated by multiplying the dioxin-like PCBs and PAHs concentrations with the toxicity equivalence factors (TEF) (Equation i) (Van den Berg et al 2006).
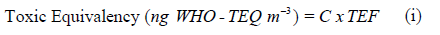
Where C is the individual concentrations of PCBs and PAHs
Health risk analysis: Health risk assessment of PAHs in the mainstream smoke of cannabis rolls was carried out using indexes such as Toxicity Equivalence Quotient (TEQ), Incremental Life Cancer Risk (ILCR), and Hazard Quotient (HQ). Calculation of TEQ, ILCR, and HQ was done using Equation (i), (ii), and (iv), respectively [23-25].

EC is the exposure concentration is calculated with Equation i, UR is the cancer unit risk, in the study ILCR is calculated using WHO and USEPA cancer Unit Risk factors. The values are 8.7 × 10-2 and 6 × 10-4) min-1 for WHO and USEPA, respectively. C is the PAH concentration based BaP, ED is exposure duration in years, in this study we made use of a minimum value of 10 years. EF is exposure frequency in days/years. It is calculated that tobacco product addicts smoke every day, which translates to 365 days/year. AT is the average exposure time in days, 25550 was adopted for calculation. EDI is the estimated daily intake of PAHs which is calculated using Equation ii. RfD (2 × 10-3) is the reference dose of Bap which is calculated from inhalation reference concentration. IR is the inhalation rate (16 m3/ day) while BW is body weight (kg).
Incremental lifetime cancer risk and hazard quotient for PCBs
Incremental Lifetime Cancer Risk (ILCR) associated with inhalation of PCBs in the indoor environment was calculated using Equation v while the non-carcinogenic associated risk was assessed using Hazard Quotient Index (HQ) Index (Equation vi) [26].
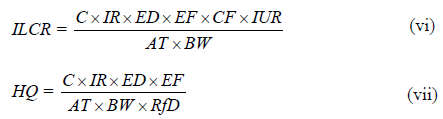
C is the PCB concentration (μg m−3). ED is exposure duration in years (52 years was adopted based on average life expectancy in Nigeria) [26]. EF is exposure frequency in day’s year −1 (350 days). IUR is the inhalation unit risk (5.7 × 10-6 μg m−3). AT is the average exposure time in days, 25550 was adopted for calculation. BW is body weight in (kg). RfD is the reference dose of PCB (3.3 × 10-5).
Result and Discussion
The concentration of PCBs in the mainstream smoke
In this study, a total of 20 PCBs were targeted: 3-chlorobiphenyl (PCB 2), 1,1'-biphenyl, 2,2',3-trichloro-(PCB 16), 1,1'-Biphenyl, 2,2',5-trichloro-(PCB 18), 1,1'-biphenyl, 4,4'-dichloro-(PCB 15), 1,1'-Biphenyl, 2,2',5,5'-tetrachloro-( PCB 52), 1,1'-Biphenyl, 2,3,3',4,4'-pentachloro-(PCB 105), 1,1'-Biphenyl, 2,2',3,4',5',6-hexachloro-(PCB 149), 1,1'-Biphenyl, 2,3,4,4',5-Pentachloro-(PCB 114), 2,3',4,4',5'-Pentachloro-1,1'-biphenyl (PCB 123), 1,1'-Biphenyl, 2,2',3,4,4',5'-hexachloro-(PCB 138), 1,1'-Biphenyl, 2,2',3,5'-tetrachloro-(PCB 44), 1,1'-Biphenyl, 3,3',4,4'-tetrachloro-( PCB 77), 1,1'-Biphenyl, 2,2',4,5,5'-pentachloro-( PCB 101), 1,1'-Biphenyl, 2,3',4,4',5-pentachloro-( PCB 118), 1,1'-Biphenyl, 2,4,4'-trichloro-(PCB 28), 1,1'-Biphenyl, 2,3,3',4,4',5-hexachloro-((PCB 156), 1,1'-Biphenyl, 3,3',4,4',5,5'-hexachloro-(PCB 169), 1,1'-Biphenyl, 2,3,3',4,4',5'-hexachloro-(PCB 157), 1,1'-Biphenyl, 2,2',3,4,4',5,5'-heptachloro-(PCB 180), 1,1'-biphenyl, 2,2',3,4,4',5,6,6'-octachloro (PCB 204).
Table 1 shows the level of PCBs observed in the analyzed marijuana samples. The observed concentration of PCBs in this study is higher than the observation of Radovanovic and Misic, (1998) and Wilson, et al., (2008) done for the mainstream smoke of the cigarette. The most predominant PCB found in the sample analyzed is PCB 15 which is 16% of the total PCBs present in the marijuana sample. Another predominant PCB in the smoke was PCB 114 with 15% of total PCBs. The PCB with the least percentage distribution is PCB 44, taking only 1% of the total amount of PCB in the sample.
Table 1: Concentration of PCBs in the Cannabis samples.
| PAHs | Mean | std | Min | Max | % of Total |
|---|---|---|---|---|---|
| PCB 2 | 0.56 | 0.22 | 0.42 | 0.59 | 2 |
| PCB 101 | 0.59 | 0.02 | 0.47 | 0.6 | 2 |
| PCB 52 | 1.31 | 0.82 | 1.32 | 1.35 | 5 |
| PCB 16 | 3.08 | 0.53 | 3.02 | 3.12 | 12 |
| PCB 77 | 2.89 | 0.65 | 2.8 | 2.9 | 11 |
| PCB 118 | 2 | 0.23 | 1.98 | 2.3 | 8 |
| PCB 18 | 2.67 | 0.12 | 2.64 | 2.78 | 10 |
| PCB 15 | 4.09 | 0.8 | 3.99 | 4.25 | 16 |
| PCB 28 | 2.19 | 0.23 | 2.11 | 2.23 | 8 |
| PCB 105 | 2.2 | 0.11 | 2 | 2.23 | 9 |
| PCB 44 | 0.33 | 0.12 | 0.3 | 0.35 | 1 |
| PCB 114 | 3.95 | 0.22 | 3.94 | 4.02 | 15 |
| ∑ PCBs | 25.86 | ||||
| ∑ dl-PCBs | 11.04 |
According to USEPA, 2015 [27], the classification of PCBs can be done based on 'dioxin-like' and 'non-dioxin- like'. The dioxin-like PCBs cause the production of dioxin- like effects [28]. The dioxin-like PCBs exhibit dioxin behaviours which cause the activation of the aryl hydrocarbon receptor and induce the multiplication of genes and in turn have biological effects (ATSDR, 2005: Adesina, 2021) [22]. In the analysed sample, four dioxin-like PCBs were observed: PCB 77, PCB 105, PCB 114, and PCB 118, with values of 2.89 ng, 2.2 ng, 3.95 ng, and 2 ng respectively. The least dioxin-like PCB in the sample is PCB 118, while the highest concentration of dioxin-like PCB is PCB 114.
Figure 1 shows the distribution of the dioxin-like PCBs found in the marijuana sample. 43% of the total PCBs found in the sample is dioxin-like, while the non-dioxin like account for 57%, according to previous research studies, Non-dioxin-like compounds can modulate the overall toxic potency of dioxin-like compounds. PCBs can also be classified based on their homolog patterns; Figure 2 shows the PCB homolog pattern in the analyzed marijuana sample. Penta-chlorinated (5-chlorinated) accounts for 33.8% of the total PCB congeners in the sample, tetra-chlorinated (4 chlorinated) accounts for 17.52%, tri-chlorinated accounts for 30.7%, bi-chlorinated (2 chlorinated) accounts for 15.81% and mono-chlorinated PCBs accounts for the least value of 2.17%.
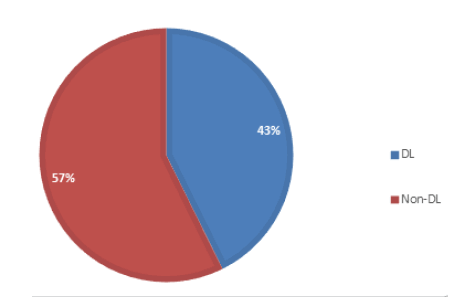
Figure 1: Distribution of Dioxin-like (DL) and Non-dioxin Like (Non-DL) PCBs in samples.
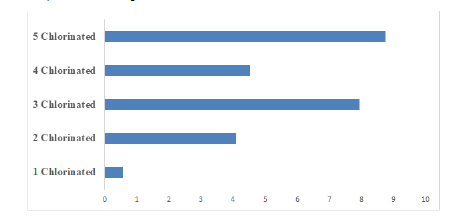
Figure 2: Polychlorinated Biphenyls homolog pattern in analyzed marijuana sample.
Concentrations of PAHs
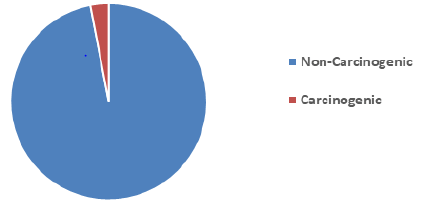
16 EPA Priority PAHs: naphthalene (Naph), acenaphthalene (Acy), acenaphthene (Ace), fluorene (Fln), phenanthrene (Phe), anthracene (Ant), fluoranthene (Flt), pyrene (Pyr), benzo[a]anthracene (BaA), chrysene (CHR), Benzo[b]fluoranthene(BbF), Benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), indeno[1,2,3-cd]pyrene (IcP), dibenz[a,h]anthracene (DhA), benzo[ghi]perylene (BgP). Table 2 shows the level of PAHs present in the marijuana sample analyzed. The most dominant PAH in the sample is Phenanthrene taking up 24% of the total amount of PAHs in the sample, Fluorene, Fluoranthene, and Acenaphthylene are present in the sample in significant quantities taking up 22%, 19%, and 17% of the total PAHs respectively, this could be due to the low molecular weight of these compounds. These results observed is consistent with Moir, et al. (2008) [29], Wei, et al., (2016) who had previously analyzed marijuana for. 2 out of 7 PAHs compounds classified to be carcinogenic PAHs were observed in the marijuana sample. The compounds are Benzo[a]Anthracene (BaA) and Chrysene (Chry). The values of BaA and Chry observed in the sample are 31.08 ng and 10.02 ng respectively. Figure 3 shows the level of Carcinogenic PAHs and non-carcinogenic PAHs in the sample, the total amount of carcinogenic and non-carcinogenic PAHs observed in the analyzed marijuana are 1322.51 ng and 41.1 ng respectively. Benzo[a]Pyrene, usually a marker for carcinogenic activity and stability of PAHs was not observed in the sample during analysis.
Table 2: Concentration of PAHs in the mainstream smoke of Cannabis.
| PAHs | Mean | std | Min | Max | % of Total |
|---|---|---|---|---|---|
| Naph | 132.93 | 0.02 | 131.1 | 133.2 | 10 |
| Acy | 226.77 | 0.03 | 225.1 | 228.1 | 17 |
| Ace | 33.61 | 0.04 | 32.2 | 34.01 | 2 |
| Fln | 305.74 | 0.06 | 305.6 | 306.1 | 22 |
| Ant | 27.54 | 0.05 | 27.43 | 28.01 | 2 |
| Phe | 321.3 | 0.03 | 320.2 | 322.2 | 24 |
| Pyr | 18.53 | 0.03 | 18.01 | 19.21 | 1 |
| Flt | 256.09 | 0.03 | 255.01 | 257.3 | 19 |
| BaA | 31.08 | 0.04 | 30.2 | 31.5 | 2 |
| Chry | 10.02 | 0.05 | 9.9 | 1.04 | 1 |
| SUM | 1363.61 |
Figure 3: Level of Carcinogenic and Non-carcinogenic PAHs.
PAHs classification can be done based on the number of rings in each compound. 2-rings PAHs (Naph), 3-rings PAHs (Acy, Ace, Fln, Ant, and Phe), and 4-rings (Pyr, Flt, BaA, and Chry) were found during marijuana analysis. Figure 4 shows the distribution of PAHs based on the number of rings, the easy formation of 2 and 3 rings PAHs is due to their low molecular weight could be the reason for their high concentration in the smoke. PAHs classification can also be by molecular weight, Low Molecular Weight (LMW), consisting of 2 and 3 rings PAHs, Middle Molecular Weight (MMW) consisting of 4 rings PAHs, and High Molecular Weight (HMW) consisting of 5 and 6 rings PAHs. LMW accounts for 77% of the total PAHs, and MMW accounts for 23%, no HMW was found in the sample.
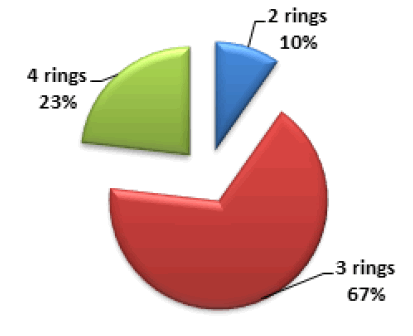
Figure 4: Distribution of PAHs according to the number of rings present in sample.
Health implication
Toxicity equivalence quotient and inhalation risk assessment of PCBs: Toxicity Equivalence Quotient (TEQ) values of the marijuana sample has a range of 0.00006 ng- 0.000289 ng TEQ with a mean value of 0.01633 ng TEQ. Inhalation Risk Assessment (IRA) was used to determine the long-term inhalation risk of PCBs from marijuana. The calculated daily inhalation exposure values ranged between 0.007347-0.03539 ng TEQ kg-1day-1. The inhalation risk assessment value calculated in this study is several times higher than the proposed Tolerance Daily Intake (TDI) by WHO of 1000 fg TEQ kg-1day-1 (WHO, 1998). This implies that continuous exposure to PCBs via marijuana smoking could cause hazardous effects on the human respiratory system. There is a dearth of literature on the analysis of mainstream marijuana smoke and its inhalation risk assessment, the IRA values calculated in this study are higher than IRA values from previous studies that worked on ambient and indoor environment tobacco smoke.
Incremental lifetime cancer risk and hazard quotient of PCBs: The determination of the Incremental probability of a person developing cancer as a result of exposure to PCBs from mainstream marijuana smoke is done by Incremental Lifetime Cancer Risk (ILCR) analysis. The ILCR values ranged between 6.98 × 10-10-1.38 × 10-9 and a mean value of 9.63 × 10-10. The values calculated are lower than the permissible limit of 1.07 × 10-6, stipulated by USEPA. The presence of other carcinogenic compounds such as PAHs and Volatile Organic Compounds (VOC) will increase the cancer risk significantly in individuals exposed to the concentration of all the carcinogenic pollutants present in mainstream marijuana smoke. The determination of the non-carcinogenic risk associated with the exposure of an individual to a pollutant is done by calculating Hazard Quotient (HQ). The calculated HQ value for PCBs smoking was 3.9981. This value is higher than 1. The health implication of PCB on a marijuana smoke addict is enormous, such individuals have a high risk of developing chronic non-cancer organ dysfunction [30,31].
Incremental lifetime cancer risk and hazard quotient of PAHs: Toxicity equivalency (TEQ) was used in the assessment of the toxicity of the individual PAHs, present in mainstream cigarette smoke. The TEQ is calculated by multiplying the level of individual PAHs by their toxicity equivalent factors (TEF) (Nisbet and LaGoy, 1992) [32]. Table 2 shows the Toxicity Equivalence (TEQ) of PAHs found in the marijuana sample analyzed, the TEQ values have a range of 0.01853 ng-3.108 ng TEQ. The ILCR value based on the WHO risk factor is calculated to be 0.02542 while the ILCR value based on the USEPA risk factor is calculated to be 1.75 × 10-5. The result shows that the ILCR value based on the WHO risk factor is higher than the permissible limit of 10-5 and the ILCR value based on USEPA is higher than the permissible limit of 10-6. These values are subject to change with an increase in exposure, frequency, and duration to marijuana smoke. The calculated HQ value was 41.74 which are several times greater than the permissible limit of 1, this indicates that the PAHs in marijuana have high cancer-causing abilities and can also can other respiratory diseases that are non-cancerous (Figure 5).
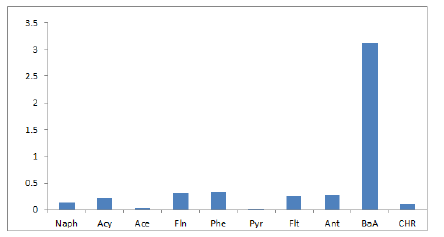
Figure 5: Mean Toxicity Equivalence of PAHs in the Marijuana sample.
Conclusion
This study determined the levels of polycyclic aromatic hydrocarbons and polychlorinated biphenyl in the mainstream smoke of some samples of Cannabis sativa. The study also went further to determine the health implications of inhaling PAHs and PCBs from marijuana. The result showed the mean Σ PCBs in the smoke was 25.86 ng while the concentration of Σ dl-PCBs was 11.04 ng. `The mean Σ PAHs in the smoke was 1363.61 ng. The ILCR values from smoking PCBs in the mainstream ranged between 6.98 × 10-10- 1.38 × 10-9 while ILCR value for exposure to PAHs 1.75 × 10-5. HQs values for exposure to PAHs and PCBs in smoke were all above 1. The study revealed both carcinogenic and non-carcinogenic risks associated with smoking marijuana.
Acknowledgements
We are grateful to all people who helped us in writing this article, especially Government Medical College, Kathua and District Police, Kathua, for providing the required information.
Conflicts of Interest
Authors have no conflict of interest to declare.
References
- B. Graves, T. Johnson, R. Nishida, P. Ryan, B. Savareear, et al. Comprehensive characterization of mainstream marijuana and tobacco smoke, Sci Rep, 10(2020):7160.
- Ajayi, O. Somefun, Recreational drug use among Nigerian university students: Prevalence, correlates and frequency of use, Plos One, 15(2020):1-14.
- K. Yeh, L. Li, F. Wania, J. Abbatt, Thirdhand smoke from tobacco, e-cigarettes, cannabis, methamphetamine and cocaine: Partitioning, reactive fate, and human exposure in indoor environment, Environ Int, 160(2022):1-17.
- C. Sempio, E. Lindley, J. Klawitter, U. Christians, R. Bowler, et al. Surface detection of THC attributable to vaporizer use in the indoor environment, Sci Rep, 9(2019):1-7.
- R. Maertens, P. White, W. Rickert, G. Levasseur, R. Douglas, et al. The genotoxicity of mainstream and sidestream marijuana and tobacco smoke condensates, Chem Res Toxicol, 22(2009):1406–1414.
- L. Ribeiro, P. Ind, Effect of cannabis smoking on lung function and respiratory symptoms: A structured literature review, NPJ Primary Care Respir Med, 26(2016):16071
- W. Ott, T. Zhao, K. Cheng, L. Wallace, L. Hildemann, Measuring indoor fine particle concentrations, emission rates, and decay rates from cannabis use in a residence, Atmos Environ: X, 10(2021):1-9.
- H. Savaki, J. Cunha, E. Carlini, T. Kephalas, Pharmacological activity of three fractions obtained by smoking cannabis through a water pipe, Bull Narc, 28(1976):49-56.
- Al-Zouabi, J. Stogner,B. Miller, E. Lane, Butane hash oil and dabbing:Insights into use, amateur production techniques, and potential harm mitigation, Subst Abuse Rehabil, 9(2018):91-101.
- T. Zhao, K. Cheng, W. Ott, L. Wallace, L. Hildemann, Characteristics of secondhand cannabis smoke from common smoking methods: Calibration factor emission rate, and particle removal rate, Atmos Environ, 242(2020):117731.
- S. Aldington, M. Harwood, B. Cox, M. Weatherall, L. Beckert, et al. Cannabis use and risk of lung cancer: A case–control study, Eur Respir J, 31(2008):280-286.
- M. Islam, M. Park, Y. Jo, X. Nguyen, S. Park, Distribution, sources, and toxicity assessment of polycyclic aromatic hydrocarbons in surface soils of the Gwangju City, Korea, J Geochem Explor, 180(2007):52-60.
- S. Gordon, M. Brinkman, R. Meng, G. Anderson, J. Chuang, et al. Effect of cigarette menthol content on mainstream smoke emissions, Chem Res Toxicol, 24(2011):1744-1753.
- World Health Organization (WHO) Regional Office for Europe. (2000). Air Quality Guidelines. Denmark
- E. Dumbili, Cannabis normalization among young adults in a nigerian city, JDI, 50(2020):1-17.
- O. Akanle, J. Adesina, O. Adebayo, Marijuana smoking among young institution of higher learning students in nigeria. Acta Criminologica, (2015):114-130. [Crossref]
- E. Dumbili, R. Hanewinkel, H. Degge, E. Ezekwe, M. Nnajiofor, Cannabis use motivations: A study of young adults in Nigeria, Drugs: Educ Prev Policy, 28(2021)1-28.
- E. Ernest, O. Chinedu, A. Ifeanyi, O. Onyeka, C. Aniobi, Heavy metal levels determination and pollution index status of smoke based and smokeless tobacco and cannabis products sold within enugu metropolis, enugu state, nigeria, Pharm Chem J, 7(2020):85-93.
- T. Nweze, C.C. Eze, F. Lange, Preserving responding in Nigerian chronic alcohol and marijuana users, Subst Use Misuse, 55(2020):1199-1202.
[Crossref] [Google Scholar] [PubMed]
- C. Gellner, D. Reynaga, F. Leslie, Cigarette smoke extract: A preclinical model of tobacco dependence, Curr Protoc Neurosci, 77(2016):1-17.
- O.A. Adesina, T.I. Olowolafe, A. Igbafe, Levels of polycyclic aromatic hydrocarbon from mainstream smoke of tobacco products and its risks assessment, J Haza Mat Adv, 5(2022):100053
- O. Adesina, Concentrations of polychlorinated biphenyls in ambient air and solid residues around a municipal solid waste open burning site, Chem Stud, 75(2021):1-8.
- M. Delikhoon, M. Fazlzadehdavilb, A. Sorooshian, B. Norouzian, M. Golaki, et al. Characteristics and health effects of formaldehyde and acetaldehyde in an urban area in Iran, Environl Pollut, 242(2018):938-951.
- S. Hazrati, R. Rostami, M. Fazlzadeh, F. Pourfarzi, Benzene, Toluene, Ethylbenzene and Xylene concentrations in atmospheric ambient air of gasoline and cng refueling stations, Air Qual Atmos, 9(2016):403-409.
- O. Adesina, A. Okewale, M. Lala, Concentration of polycyclic aromatic hydrocarbons in the soil around a university power generating set, RJEES, 3(2018):374-379.
- USEPA (1999) Polychlorinated Biphenyls (PCBs) Update: Impact on fish advisories. United States environmental protection agency
- United States Environmental Protection Agency, USEPA (2011). Exposure factors handbook (Final). Washington
- E. Aganbi, C. Iwegbue, M. Bice, Concentrations and risks of polychlorinated biphenyls (PCBs) in transformer oils and the environment of a power plant in the Niger delta, Nigeria, Toxicol Rep, 15(2019):933-939
- D. Moir, W. Rickert, G. Levasseur, Y. Larose, R. Maertens, et al. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions, Chem Res Toxicol, 21(2008):494-502
- M. Martinasek, J. McGrogan, A. Maysonet, A systematic Review of the respiratory effects of inhalational marijuana, Respir Care, 61(2016):1543-1551
- B. Fisher, C. Russell, P. Sabioni, W. Brink, B. Le Foll, et al. Lower-Risk Cannabis Use Guidelines: A comprehensive update of evidence and recommendations. Am J Public Health, 107(2017):e1-e12.
- Nisbet, P. LaGoy, Toxic equivalency factors (TEFs) for Polycyclic aromatic hydrocarbons (PAHs), Regul Toxicol Pharmacol, 16(1992):290-300.
Copyright: © 2022 Olusola Adedayo Adesina, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.