Research Article: Journal of Drug and Alcohol Research (2025) Volume 14, Issue 4
Gradenigo Syndrome, Neurocysticercosis, Drug Therapy: Case Report and Systematic Review
Lourdes de Fatima Ibanez Valdes1 and Humberto Foyaca2*2Department of Neurology, Nelson Mandela Academic Hospital, Walter Sisulu University, South Africa
Humberto Foyaca, Department of Neurology, Nelson Mandela Academic Hospital, Walter Sisulu University, South Africa, Email: humbertofoyacasibat@gmail.com
Received: 03-Jun-2025, Manuscript No. JDAR-25-166288; Editor assigned: 05-Jun-2025, Pre QC No. JDAR-25-166288 (PQ); Reviewed: 19-Jun-2025, QC No. JDAR-25-166288; Revised: 04-Aug-2025, Manuscript No. JDAR-25-166288 (R); Published: 11-Aug-2025, DOI: 10.4303/JDAR/236443
Abstract
Introduction: A quite rare and dangerous side effect of chronic otitis media is Gradenigo’s Syndrome (GS). It is usually characterized by three symptoms: Ipsilateral abducens nerve palsy, which is caused by the spread of the infectious process from the middle ear to the petrous apex of the temporal bone; facial pain; and purulent otorrhea, which is usually caused by petrous apicitis as a consequence of acute otitis media.
Neurocysticercosis (NCC) is parasitic disease caused by the larva stage of the pig tapeworm Taenia solium when infect the brain, optic nerve and the spinal cord.
Methods: We searched the medical literature, following the guidelines outlined in the PRISMA statement. From 01st, January 1944 to 31st, January 2025, the authors searched the scientific databases, Scopus, Embassy, Medline, and PubMed Central using the following searches: “Gradenigo’s syndrome” OR “Petrous apicitis” OR “Otitis media complications” OR “Abducens nerve palsy” OR “Petrositis”, OR “Cavernous sinus syndrome” OR “Trigeminal nerve” OR “Trigeminal neuralgia”
Results: After screening the full‐text articles for relevance, 61 articles were included for final review. However, no article was found when we searched for GS/NCC/responding to drug therapy.
Conclusions: As far we know, this is the case report about comorbidity of NCC and GS. The association of GS and NCC is by coincidence and there not relationship between both pathogens. The local venous system plays an important role in the pathogenesis of GS complications. The broadspectrum IV antibiotic is the treatment of choice.
Keywords
Gradenigo syndrome; Petrous apicitis; Otitis media complications; Retroâorbital pain; Mastoiditis; Skull base infections; Abducens nerve palsy; Petrositis
Introduction
A quite rare and dangerous side effect of chronic otitis media is Gradenigo’s Syndrome (GS). It is usually characterized by three symptoms: Ipsilateral abducens nerve palsy, which is caused by the spread of the infectious process from the middle ear to the petrous apex of the temporal bone, causing inflammation and pressure on the abducens and some branches of the trigeminal nerves, primarily when the petrous apex is pneumatized; purulent otorrhea, which is usually caused by petrous apicitis as a consequence of acute otitis media; and facial pain [1]. Because the traditional triad is so rare, the diagnostic workup is difficult.
Recently, Ibrahim et al. found 80 cases of GS in 190 patients with a confirmed diagnosis of Petrous Apicitis (PA) and 10% of those patients were infected by Fusobacterium necrophorum. These authors established that it is brought on by an infection that spreads to the petrous apex of the temporal bone via the Dorello canal, impacting the trigeminal ganglion and abducens nerve (Meckel’s cave). It causes ipsilateral abducens nerve palsy and excruciating pain when the first and second branches of the trigeminal nerve are irritated.
Italian otorhinolaryngologist Giuseppe Gradenigo documented this clinical experiment in 1904. He listed a number of symptoms, such as pain in the trigeminal nerve distribution, paralysis of the abducens nerve, and suppurative otitis media [2,3].
Meningoencephalitis, cerebral abscess, empyema, and venous sinus thrombosis with compression of the nearby Internal Carotid Artery (ICA) resulting in cerebral infarction are among the consequences that can occasionally be observed [1]. In 1918, Gore MR established that the mortality rate was around 2.6% [4].
The most common microbial infections found in patients presenting PA/GS are Pseudomonas (37%) and Staphylococcus aureus (32%). Therefore, antipseudomonal and anti-staphylococcal antibiotics should be considered in these cases [1,5].
Petrous apicitis can be caused by several pathogens, including Staphylococcus, Streptococcus pyogenes and Streptococcus pneumoniae, while Pseudomonas aeruginosa is the most common. According to Tao et al., a case of pediatric Gradenigo syndrome was successfully treated with antibiotics that were suitably broad-spectrum. They described a 5-year-old who was not responding to ciprofloxacin-dexamethasone drops. Following successful treatment with empiric intravenous ceftazidime and cefazolin, he healed completely without the need for surgery after 16 weeks [5].
Taenia solium Taeniasis/Cysticercosis (TSTC) is a preventable foodborne, zoonotic, neglected tropical disorder predominately seen in persons living or visiting low and middle-income endemic countries, At the same time, Neurocysticercosis (NCC) is the consequence of invasion by the same cestode of the brain, optic nerves or the spinal cord which is identified as the larva form of the pig tapeworm Taenia solium (Ts). The most typical clinical features of this condition are Epilepsy (EP), Epileptic Seizures (ES) and headache, among other problems.
Pigs act as intermediate hosts of cysticercosis while humans are definitive hosts. Taeniasis, i.e., intestinal infection with mature T. solium in the human host, occurs after ingesting undercooked pork meat infected with the larval stage (porcine cysticercosis). Cysticercosis in human beings occurs after humans ingest T. solium eggs or proglottids. NCC is the principal aetiology of secondary epilepsy in endemic areas [6-10]. ES and Ep are the most common symptoms of Cerebral Cysticercosis (CC). In the past, we performed more than a dozen epidemiological studies in rural areas around Mthatha, confirming that CC, also named Neurocysticercosis (NCC), is the primary aetiology of secondary epilepsy. Most ES and Ep respond well to first-line Antiepileptic Drugs (AED) and Antiseizure Medication (ASM) [11-20]. The most prescribe ASM is benzodiazepine, and the commonest used AED are valproic acid and carbamazepine [21-24].
This study’s primary goal is to respond to the following research inquiries: How often is GS associated with NCC? What is the most likely relationship between GS and cavernous sinus syndrome?
Materials and Methods
Search strategy
From 01 January 1974 to 31 January 2025, we searched the databases Embassy, Medline, Scopus, and PubMed Central using the following searches: “Gradenigo’s syndrome” OR “Petrous apicitis” OR “Otitis media complications” OR “Abducens nerve palsy” OR “Petrositis”, OR “Cavernous sinus syndrome” OR “Trigeminal nerve” OR “Trigeminal neuralgia”. We searched the medical literature following the PRISMA guidelines. After removing duplicates, two reviewers (LDFIV and HFS) from each side screened titles and abstracts and evaluated the full texts of eligible articles based on the proposed inclusion criteria. Any disagreement between the reviewers involved in the literature search was resolved through discussion with all authors to reach a consensus.
Selection criteria
The following manuscripts were included in the systematic review:
Articles on Isaac syndrome, neuropathic pain, and Schwann cell disorder in ISA/NP, with detailed pathogenesis and/or drug therapy.
Exclusion criteria were as follows:
• Inaccessibility to full text.
• Articles with unclear pathogenesis.
• Lack of relevant clinicopathological data.
• Non-original studies (i.e., editorials, letters, conference proceeding, book chapters).
• Animal model studies.
• Non-/Spanish/Portuguese/English studies. The papers were thoroughly assessed, and duplicates were looked for.
Data extraction and quality assessment
All selected data were tabulated in an electronic Excel database. That information included pathogenesis, drug management, initial clinical presentation, evaluation of NP/IS after treatment, follow-up, and status at the latest evaluation. The studies’ quality was categorized as good, poor, fair, or reasonable, in agreement with the National Institutes of Health criteria. Two reviewers (LDFIV and HFS) independently evaluated the articles, and the discussion resolved disagreements.
Statistical analysis
The primary objective of this study was to evaluate whether DD’s pathogenesis/drug management differs significantly among different therapies. Without a comprehensive reference for the total number of DD cases, the prevalence of DD responding to drug therapy with associated autonomic manifestations was searched through a comprehensive review.
Statistical analyses were performed using XLSTAT (add-on for Microsoft Excel, version 2021.4.1, Addinsoft SARL) and RStudio (version 4.3.1, https://www.rstudio.com/). Variations in continuous variables were assessed using the Mann-Whitney U-test. We presented descriptive statistics for continuous variables as median (95% Confidence Interval (95% CI)). All situations were evaluated using the Kaplan-Meier method to identify relevant prognosticators. A model of multivariable Cox proportional hazards with a priori selection of covariates was used to check for independent prognostic effects.
Results and Discussion
A total of 514 titles were selected from the literature. After removing duplicates and excluding records. 61 relevant articles were examined. Twenty-three studies were unavailable for retrieval. After including six additional articles identified from citation searching, 43 were excluded for several reasons. A total of 61 records were identified from these searches. The resulting study titles were exported to Excel, and publications without including/ excluding criteria duplicates were removed, leaving 22 unique titles. We screened all remaining unique full-text articles, abstracts, and titles for eligibility. Titles were typically excluded if unavailable in Spanish, Portuguese, or English or irrelevant to GS. Articles relevant to 53 included the clinical presentation, management, treatment, and pathogenesis. The authors then screened these articles by abstract. After further review, abstracts were excluded if they were irrelevant to the topic, outdated, or unavailable in Spanish, Portuguese, or English. No articles were for full-text review because they did not report comorbidity of GS and NCC. Finally, when we searched for GS/NCC, no article was identified.
Series description and differences among groups
All the selected studies were relevant to the subject of this systematic review. None were randomized controlled trials or prospective studies; all the articles included were case reports and case series. The total number of patients presenting with documented GS/NCC was zero.
Median age was 14.7 (range 5-71) with significant differences between age groups (p<0.001). We did not find remarkable variations in gender (p=0.064), although males presenting GS were noticeably more frequent and slightly more prevalent.
A 39-old known chronic epileptic male patient secondary to calcified NCC well controlled under valproic acid 600 mg po twice a day over the past seven years. He came to the neurology OPD complaining of recurrent tonic-clonic generalized epileptic seizures for the past four days, with no response to the previous AED. He said that five days prior, he presented with severe generalized headache, left yellow-green-coloured otorrhea, facial pain, numbness, weakness of muscles of mastication, mild fever and otalgia. He attended the ENT clinic, where ciprofloxacin-dexamethasone ear ointment was prescribed.
However, the frequency of epileptic attacks increased, and the patient complained of horizontal binocular diplopia, worse on left gaze and excruciating left-side facial pain. He denied experiencing any recent trauma, tooth issues, or prior bouts of comparable symptoms.
On examination, he was fully alert and had typical vital signs. A left tympanic membrane perforation (anterior superior quadrant) with overt otorrhea, mild mastoid tenderness, and trace hearing reduction on the left side were confirmed. Neurological examination demonstrated left abducens nerve palsy. Given the severity of the symptoms, indications, and suspicion of an infectious process overlay, laboratory testing were conducted. The total blood count revealed a white blood cell count of 14,500/μL, which is within the reference range of 4,000–11,000/μL, indicating mild leucocytosis. The erythrocyte sedimentation rate was raised to 55 mm/h (reference range: 0-20 mm/h), and the C-reactive protein was raised to 35 mg/L (reference range: <5 mg/L). An infection of the central nervous system was ruled out by the unremarkable results of the cerebrospinal f luid investigation. The microorganisms were confirmed by blood cultures, and broad-spectrum antibiotics were started right away.
Laboratory investigations exhibited a normal white blood count, marked platelet elevation (691 × 109/L), and mildly raised C-reactive protein (12 mg/L). A rhinovirus/ enterovirus swab was positive. Blood cultures were negative after 48 hours. However, the ear culture was found to be unprocessed after 3 days, as it was lost in transit to the laboratory. Repeat ear swabs and culture were negative after a further 48 hours.
Magnetic resonance confirmed bilateral multiple calcifications in the cerebral parenchymal involving the grey and White matter, clival osteomyelitis, otomastoiditis, and coalescent left petrous apicitis. Finally, there was asymmetric enhancement involving the left carotid canal with moderate narrowing of the left internal carotid artery without signs of cerebral venous sinus thrombosis.
To eradicate the bacterial pathogens causing mastoiditis and petrous apicitis, the patient was given intravenous ceftriaxone (2 g every 12 hours) and metronidazole (500 mg every 8 hours). To remove the purulent substance from the middle ear, a myringotomy was carried out after consulting with the otolaryngology specialists.
Comments and final remarks
All publications on Gradenigo syndrome, petrous apicitis, the number of cases reported with otitis media, their age and sex are included in Table 1, which is self-explanatory [25-86].
| First author | Year | PA cases | GS | OM | Age | Sex |
|---|---|---|---|---|---|---|
| Bridgewater [25] | 1944 | 1 | Yes | Yes | 9 | F |
| Ogilvie [26] | 1945 | 2 | Yes | Yes | 10 | F |
| Yes | Yes | 31 | M | |||
| Horowitz [27] | 1948 | 2 | Yes | Yes | 14 | F |
| Yes | Yes | 30 | F | |||
| Eby [28] | 1961 | 3 | Yes | Yes | 21 | M |
| No | Yes | 44 | M | |||
| Yes | Yes | 38 | F | |||
| Gillespie [29] | 1962 | 1 | Yes | – | – | – |
| Hiranandani [30] | 1967 | 1 | Yes | Yes | 30 | M |
| Horn [31] | 1996 | 1 | Yes | Yes | 10 | F |
| Murakami [32] | 1996 | 1 | Yes | Yes | 8 | F |
| Minotti [33] | 1999 | 2 | Yes | Yes | 47 | F |
| Yes | Yes | 36 | F | |||
| Jagadeesan [34] | 2002 | 1 | Yes | Yes | 4 | F |
| Piron [35] | 2003 | 1 | Yes | Yes | 5 | F |
| Park [36] | 2003 | 1 | Yes | Yes | 8 | F |
| Finkelstein [37] | 2003 | 1 | Yes | Yes | 12 | M |
| Sethi [38] | 2005 | 1 | Yes | Yes | 11 | F |
| Visosky [39] | 2006 | 4 | Yes | Yes | 8 | M |
| Yes | Yes | 14 | M | |||
| No | Yes | 66 | F | |||
| No | Yes | 84 | M | |||
| Hafidh [40] (NB: description of individual patients not provided) | 2006 | 3 | – | – | – | – |
| Rossor [41] | 2011 | 1 | Yes | Yes | 11 | F |
| Pollock [42] (NB: description of individual patients not provided) | 2011 | 7 | Yes | Yes | – | M=5, F=2 |
| Kong [43] | 2011 | 1 | Yes | Yes | 10 | M |
| Jacobsen [44] | 2012 | 1 | Yes | Yes | 3 | M |
| Colpaert [45] | 2013 | 1 | Yes | Yes | 12 | F |
| Heshin-Bekenstein [46] | 2013 | 2 | Yes | Yes | 3 | M |
| Yes | Yes | 5 | F | |||
| Chen [47] | 2014 | 4 | Yes | Yes | 64 | F |
| Yes | Yes | 33 | F | |||
| Yes | Yes | 58 | M | |||
| Yes | Yes | 45 | M | |||
| Choi [48] | 2014 | 1 | Yes | Yes | 8 | F |
| Plodpai [49] | 2014 | 1 | Yes | Yes | 63 | M |
| Valles [50] | 2014 | 1 | Yes | Yes | 36 | F |
| Jensen [51] | 2016 | 4 | Yes | Yes | 5 | M |
| Yes | Yes | 46 | F | |||
| Yes | Yes | 70 | F | |||
| Yes | Yes | 13 | M | |||
| Kazemi [52] | 2017 | 1 | Yes | Yes | 33 | M |
| Jensen [53] | 2017 | 1 | Yes | Yes | 9 | F |
| Vitale [54] | 2017 | 1 | Yes | Yes | 8 | F |
| Dorner [55] | 2017 | 1 | Yes | Yes | 5 | M |
| Taklalsingh [56] | 2017 | 1 | Yes | Yes | 58 | M |
| Ghani [57] | 2017 | 1 | Yes | Yes | 7 | M |
| Solms [58] | 2017 | 1 | Yes | Yes | 10 | M |
| Shapiro [59] | 2017 | 1 | Yes | Yes | 11 | F |
| Gadre [60] (NB: description of individual patients not provided) |
2017 | 44 | 6 (13.6%) | 27 (54.5%) | 8–76 (Mean=39.2) | M=23, F=21 |
| Brunet-Garcia [61] | 2018 | 1 | Yes | Yes | 41 | M |
| Al-juboori [62] | 2018 | 1 | Yes | Yes | 61 | M |
| Bozan [63] | 2018 | 1 | Yes | Yes | 9 | F |
| Özkaçmaz [64] | 2019 | 1 | Yes | Yes | 14 | F |
| Rossi [65] | 2019 | 1 | Yes | Yes | 4 | F |
| Athapathu [66] | 2019 | 1 | Yes | Yes | 6 | M |
| Brambilla [67] | 2019 | 1 | Yes | Yes | 8 | – |
| Savasta [68] | 2019 | 1 | Yes | Yes | 11 | M |
| McLaren [69] | 2020 | 1 | Yes | Yes | 5 | F |
| Patel [70] | 2020 | 2 | Yes | No | 49 | F |
| No | Yes | 46 | M | |||
| Hodges [71] | 2020 | 1 | Yes | Yes | 24 | M |
| Demir [72] | 2020 | 1 | Yes | Yes | 7 | M |
| Chandran [73] | 2020 | 2 | Yes | Yes | 54 | F |
| Yes | Yes | 23 | F | |||
| Guimaraes [74] | 2021 | 1 | Yes | Yes | 63 | F |
| Sattarova [75] | 2021 | 1 | Yes | Yes | 65 | M |
| Malic [76] | 2021 | 1 | Yes | Yes | 5 | F |
| Liu [77] | 2021 | 1 | Yes | Yes | 35 | F |
| Quesada [78] | 2021 | 1 | Yes | Yes | 14 | F |
| Bonavia [79] | 2022 | 1 | Yes | Yes | 14 | F |
| Jin [80] | 2022 | 1 | Yes | Yes | 78 | M |
| Chan [81] | 2023 | 1 | Yes | Yes | 5 | M |
| Brenda [5] | 2024 | 1 | Yes | Yes | 5 | M |
| Horache [82] | 2024 | 1 | Yes | Yes | 15 | M |
| Ali [83] | 2024 | 1 | Yes | Yes | 38 | F |
| Mammarella [84] | 2024 | 1 | Yes | Yes | 71 | M |
| Kim [85] | 2024 | 1 | Yes | Yes | 11 | M |
| Guennouni E [86] | 2025 | 1 | Yes | Yes | 8 | M |
Table 1: A summary of the literature reviewed
We did not find any reports on GS associated with NCC in our systematic review of the medical literature, probably because the comorbidity of GS and calcified NCC happens by coincidence only and GS is a very rare disorder despite that there is not a pathophysiological relationship between the Taenia solium and the bacterial infections causing OM and its complications, including GS. On the other hand, in South Africa, active NCC is already eradicated, although some patients presenting with epilepsy secondary to calcified NCC are still around seeking for medical attention [87].
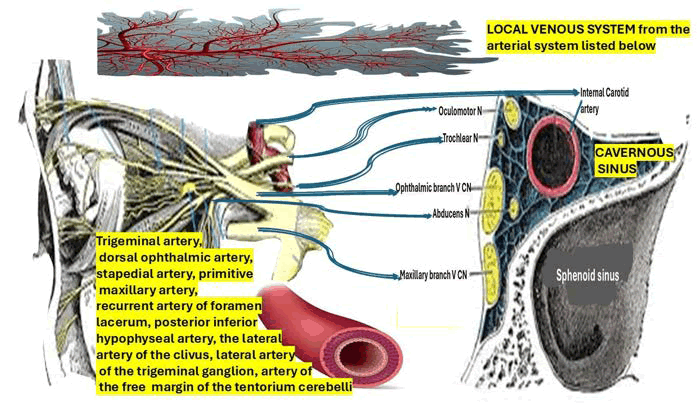
To properly understand GS’s pathophysiology, it is mandatory to be familiar with the most important aspects related to the affected cranial nerve in this syndrome. One of the most affected cranial nerves in GS is the Abducens Nerve (AN). At the pontomedullary junction, which is typically situated medially and caudally to the facial and vestibulocochlear nerves, the AN fibers depart the brainstem after leaving the sixth nerve nucleus from the dorsal pons and ventral to the fourth ventricle. After passing through the SAS, the AN continues on toward the clivus, reaching the upper border of the petrous bone tip of the temporal bone. The nerve is particularly vulnerable to infection by the aforementioned bacteria, mastoiditis, otitis, and other conditions at this location of the petrous bone. Despite its location, the AN nerve is susceptible to stretching with changes in intracranial pressure in Dorello’s canal, a fibrous boneless sheath that surrounds it after it passes the clivus. For this reason, abducens nerve palsy is not regarded as a localized neurological symptom. To innervate the lateral rectus muscle, AN leaves Dorello’s canal, enters the CS, and then follows the ICA before entering the orbital cavity through the superior orbital fissure. The second one is the trigeminal nerve, responsible for the facial pain, as we report in our case and it’s represented in Figure 1.
Figure 1: Anatomical illustration showing cranial nerves, arterial branches, and the cavernous sinus in relation to the sphenoid sinus and venous system
Brief comments on Cavernous Sinus (CS) syndrome as a complication of Gradenigo syndrome
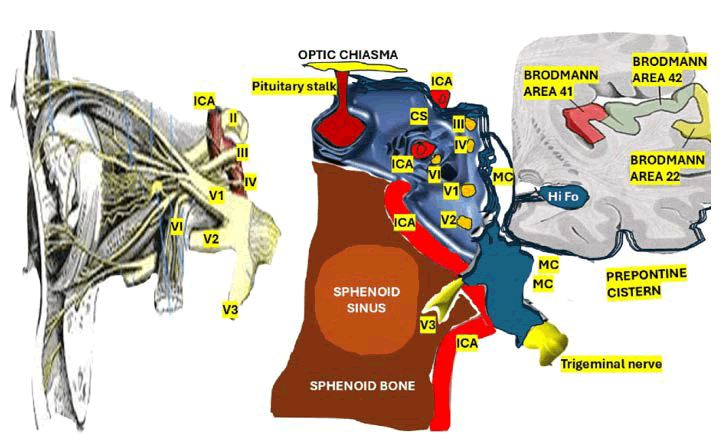
The CS is a tripartite venous osteomeningeal compartment that is adjacent to the internal carotid artery, the pituitary gland, the optic tracts, and cranial nerves III, IV, V, and VI (Figure 2).
Figure 2: Illustration showing cranial nerves, internal carotid artery, sphenoid structures, and Brodmann areas in relation to the cavernous sinu
Drawing from research conducted by Lasjaunias and Moret et al. [88-90], we can emphasize that the Cavernous Sinus (CS) is supplied by four arteries: The trigeminal artery, the dorsal ophthalmic artery, the stapedial artery, and the primitive maxillary artery. Following birth, the CS is supplied by the posterior inferior hypophyseal artery, the recurrent artery of foramen lacerum, the lateral artery of the clivus, also known as the dorsal meningeal artery, the lateral artery of the trigeminal ganglion, the lateral artery of the trigeminal ganglion, and the artery of the free margin of the tentorium cerebelli (Figure 1). On the other hand, the ocular globe and optic nerve are supplied by the dorsal ophthalmic artery. The internal maxillary artery, maxillary mandibular, and middle meningeal artery [91] will all get contributions from the stapedial artery. After the ocular division, the adult trigeminal artery leaves the basilar system and travels medially to the fifth cranial nerve before joining the internal carotid close to the carotid syphon in the CS. We hypothesized that some cases presenting CS syndrome can be explained by hematogenous spread of the infection through the trigeminal veins into the CS.
A proper understanding of the complexity of the CS’s blood circulation will serve to comprehend better the pathophysiology of the CSS from GS hematogenous spreading complications.
The anatomical properties of the trigeminal (Gasserian) ganglion, which are dural and covered in a fine reticular membrane attendant to the branches that assist the clinical symptoms of face pain described in our case, led us to speculate that the GS’s pathophysiology is influenced by these features. The sympathetic plexus fibres passing from the ICA margin to the abducens nerve play an important role in this mechanism.
We hypothesized that the involvement of the local venous system (Figure 1) plays the principal role in the complication of GS presenting as CSS. The anterior/ inferior compartment (below the intracavernous carotid, lateral to the clival region), the medial venous compartment (between the internal carotid and the pituitary), and the posterosuperior compartment (between the internal carotid artery and the posterior roof of the sinus) are the three local spaces surrounding the CS that are known to be susceptible to thrombophlebitis stemming from complicated GS.
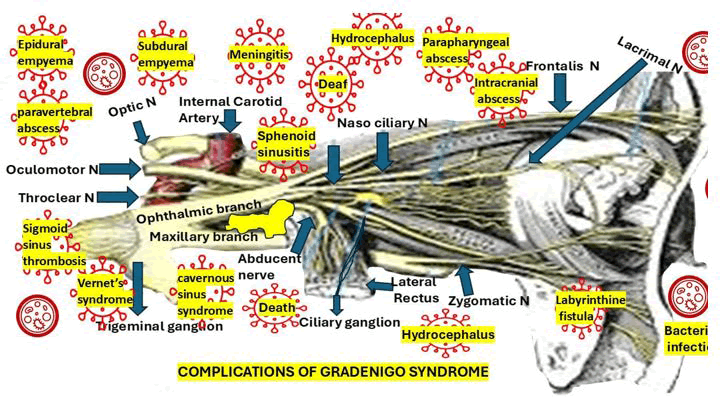
We hypothesized that CSS might be a complication of sphenoid sinusitis (Figure 3) from a complicated GS by direct or hematogenous extension. The most common com plications of GS are represented in Figure 3.
Figure 3: Anatomical depiction of Gradenigo syndrome showing cranial nerve involvement and severe complications such as meningitis, abscesses, and hydrocephalus
In our opinion, when there is a direct spread of the bacterial infection from GS through venous channels and/or damaged sinus wall of the CS, which can trigger the thrombus formation or thrombophlebitis, resulting in internal carotid artery vasospasm and extensive engorgement of the superior ophthalmic vein. We have theorized that the connecting venous vascular channels mainly contribute to the previously cited mechanism. Although it is not represented in Figure 1, we thought that the list of GS problems caused by bacterial migration into the CSF should include meningoencephalitis.
There are many stages of superior orbital fissure syndrome that can result from sphenoidal sinusitis, which is characterized by Horner’s syndrome (mainly with myosis), proptosis, ophthalmoplegia, orbital cellulitis, facial pain or hyperesthesia, and orbital apex syndrome involving the optic nerve, as has been reported by other authors [92].
Another anecdotal way of spreading bacterial infection from complicated GS is through the pterygoid plexus veins and pterygomaxillary space into the CS [93].
There is a natural mouth-shaped aperture within the petrous apex’s meningeal dura propria and periosteal layers measuring 4 × 9 mm wide ant its opening and 15 mm in length in the medial portion of the meddle cranial fossa known as Meckel’s cave which conduit for the proximal rootlets of the trigeminal nerve, connects the CS to the prepontine cyst of the posterior fossa that house the Gasserian ganglion [94].
Several pathological conditions, such as inflammatory processes, congenital disorders, infectious diseases, and vascular or neoplastic lesions, may involve Meckel’s cave.
Brief comments on the infection causing GS and its drug therapy
Before discussing about the aetiological infectious of GS, we like to mention the most relevant differential diagnosis of GS as it’s listed in Table 2.
| Cholesteatoma, or implantation of epithelium by trauma to the tympanic membrane, can grow to a size that puts necrotic pressure on the petrous bone. Jackler RK, Parker DA. Radiographic Differential Diagnosis of Petrous Apex Lesions. The American Journal of Otology. 1992;13(6):562-573. |
| Tolossa-Hunt syndrome, Lemierre’s syndrome. Kenza Horache, Manal Jidal, Ibtissam ElOuali, Rachida Saouab, Jamal Elfenni. Insights into Gradenigo syndrome: Case presentation and review. Radiol Case Rep. 2024 Sep 3;19(11):5442–5446. |
| Petrous effusion is fluid that drains into the air cells of the petrous temporal bone. This may be asymptomatic but can also form cysts. Jackler RK, Parker DA. Radiographic Differential Diagnosis of Petrous Apex Lesions. The American Journal of Otology. 1992;13(6):562-573. |
| Osteomyelitis may develop following chronic infection of the external ear, middle ear, mastoid, or petrous apex. Jackler RK, Parker DA. Radiographic Differential Diagnosis of Petrous Apex Lesions. The American Journal of Otology. 1992;13(6):562-573. |
| Neoplasms such as meningioma, intracranial plasmacytoma, chondroma and chondrosarcoma of the petrous temporal bone are very rare. Jackler RK, Parker DA. Radiographic Differential Diagnosis of Petrous Apex Lesions. The American Journal of Otology. 1992;13(6):562-573. |
| Cholesterol granuloma occurs when red blood cells and other tissue break down, releasing cholesterol to form crystals that induce an inflammatory response. These can form granulomas that destroy portions of the temporal bone. Jackler RK, Parker DA. Radiographic Differential Diagnosis of Petrous Apex Lesions. The American Journal of Otology. 1992;13(6):562-573 |
Table 2: Differential diagnosis
GS is a condition that can be caused by different types of bacterial infections, includes strains of Pseudomonas aeruginosa and Streptococcus/Staphylococcus [86]. There are several drug therapies able to control the most common bacterial infection leading to a petrous apicitis, such as ceftriaxone, vancomycin, metronidazole, benzylpenicillin, cefotaxime, clarithromycin, amoxicillin-clavulanate, and teicoplanin, despite none of them having reliable activity against Pseudomonas aeruginosa. Same authors have suggested that topical antibiotic therapies can be effective against Pseudomonas aeruginosa, but this drug therapy alone would not be sufficiently compelling to control intracranial infections [95].
Failure to properly treat GS may result in many complications as listed in Figure 2 or an inferior/fatal outcome. Therefore, we recommended a therapeutic program with sufficient coverage of all potential common pathogens, mainly non-confident culture studies or clinical evidence of skull-base involvement.
Other authors recommend administering intravenous antibiotics and anticoagulants, reporting radiological improvement and complete resolution of symptoms [82].
Other investigators prefer combining fluoroquinolones, vancomycin, and second or third-generation cephalosporins [96], only suggesting surgical debridement approaches for cases presenting osteomyelitis or abscess formation [97].
Notably, the petrous apex is close to important neurovascular systems, such as Meckel’s Cave (MC) and the abducens nerve, contributes to the characteristic clinical features of GS, including facial pain, otalgia, and abducens nerve palsy [83]. It also makes it difficult to get a swift response to the drug therapy, mainly in cases of septic collection and dural enhancement around Meckel’s cave, which will be documented below. Ali, et al. [83] also recommend requesting the otolaryngology team’s support to perform a myringotomy to drain purulent material from the middle ear.
However, a 71-year-old man with a history of chronic otitis media and poorly managed type 2 diabetes mellitus presented with a GS by COM infection by Pseudomonas aeruginosa and delayed onset of abducens nerve palsy. He was treated with broad-spectrum antibiotics (IV beta lactam) but showed no clinical improvement, according to Mammarella et al. [84].
In a 2022 systematic assessment of 134 PA patients, Talmor, et al. discovered that only 28 instances (20.9%) had the traditional GS triad of retro-orbital or facial discomfort, otorrhea, and abducens palsy [97,98]. These authors also found that Klebsiella pneumoniae is another pathogen frequently associated with GS, apart from two cases presenting tubercular petrositis, two infected by Fusobacterium necrophorum, two by Candida parapsilosis and one by nontuberculous Mycobacteria.
The many diagnostic classifications of GS, which are separated into three groups, do not correlate with the kind of infection or response to therapeutic drugs: PA, abducens nerve palsy, OM, and trigeminal nerve pain are signs of incomplete presentation; PA, abducens nerve palsy, and OM or trigeminal nerve pain are signs of mimic presentation; and PA, abducens nerve palsy, OM, and trigeminal nerve pain are signs of classic presentation. The diagnostic categories were proposed by McLaren and colleagues in 2020 [99].
Another reported case was treated with oral amoxicillin/ clavulanate (Augmentin) with a good response. The same authors recommend similar broad-spectrum IV antibiotics, including cefotaxime, imipenem, ceftriaxone, or piperacillin/tazobactam, for at least 2-3 weeks. For patients presenting with an associated osteomyelitis, they suggest up to 6 weeks of treatment. They also recommend adding metronidazole to cover potential anaerobic organisms [85].
Patients not responding to drug therapy may present a spreading of the infection to nearby structures, leading to many types of complications, as we mentioned before, includes Horner’s syndrome-related extension to the sympathetic plexus and involvement of the jugular foramen [99].
The appropriate medication therapy for GS is still up for debate because of its rarity and clinical variability. Drug therapy is unquestionably important, but each case must be evaluated individually based on the patient’s characteristics and the diagnostic category in order to determine the best course of action [100]. However, everybody agrees that broad-spectrum antibiotics are required to control infectious processes long-term (McLaren), primarily for all instances with abscesses that are situated in areas that are difficult to access surgically due to potential surgical complications or surgical limits [101-103].
The 8-year-old boy reported by Guennouni et al. [86] was infected by Pseudomonas aeruginosa and responded well to IV ciprofloxacin at 15 mg/kg/day for 2 weeks, followed by oral therapy. The same investigators recommend high dose systemic and local antibiotic therapy, which should be extended over a prolonged period, even if there is a sustainable improvement after a shorter drug therapy.
Table 2 shows all differential diagnoses and conditions that might mimic GS without solely originating from PA; here, he only mentions those related to inflammations, prothrombotic states, and coagulopathies [82].
Conclusion
Finally, we conclude that, it is the first reported case about comorbidity of NCC and GS. The local venous system plays an important role in the pathogenesis of GS complications. The broad-spectrum IV antibiotic is the treatment of choice.
Author Contributions
Both investigators have read and agreed to the published version of the manuscript.
Conflict of Interest Statement
The authors declare they have no conflicts of interest.
Funding Information
The authors received no funds to perform the present research.
Ethics Statement
The study was conducted using the principles of the Helsinki Declaration, the Italian and US privacy and sensitive data laws, and the internal regulations for retrospective studies of the Otolaryngology Section at Padova University and Brescia University.
Informed Consent Statement
We obtained the informed consent from the case involved in the study.
Data Availability Statement
The corresponding author will make the raw data supporting this article’s conclusions available upon request.
Acknowledgments
The authors thank Prof Thozama Dubula, Head of Department on Internal Medicine and Therapeutic for his unconditional support in the management of this patient.
References
- M. M. Nahvizadeh, S. Akhavan, S. Arti, L. Qaraat, N. Geramian, et al. A review study of substance abuse status in high school students, Isfahan, Iran, Int J Prev Med, 5(2014):S77.
[Crossref] [Google Scholar] [PubMed]
- Z. Ibrahim, S. FoxâLewis, J. A. Correia, Fusobacterium necrophorum an underrecognized cause of petrous apicitis presenting with Gradenigo syndrome: A case report, Am J Case Rep, 25(2024):e942652â1–e942652â30.
[Crossref] [Google Scholar] [PubMed]
- G. Gradenigo, About circumscriptive leptomeningitis with spinal symptoms and about paralysis of the abducens nerve of otic origin, Arch Ohrenheilkd, 62(1904):255–270.
- M. Motamed, Gradenigo's syndrome, Postgrad Med J, 76(2000):559-560.
- M. R. Gore, Gradenigo’s syndrome: A review, Ann Med Health Sci Res, 8(2018):220–224.
- B. K. Tao, F. Alotaibi, A. McAlpine, Ceftazidimeâcefazolin empiric therapy for pediatric Gradenigo syndrome, Ann Otol Rhinol Laryngol, 134(2024):234–237.
[Crossref] [Google Scholar] [PubMed]
- H. Foyaca-Sibat, L. Ibanez-Valdes, Pseudo seizures and epilepsy in neurocysticercosis, Electron J Biomed, 2(2003):20-29.
- H. Foyaca-Sibat, A.H. Del Rio-Romero, L. Ibanez-Valdes, E. Vega-Novoa, Neuroepidemiological survey for epilepsy and knowledge about neurocysticercosis at Ngqwala location, South Africa, Internet J Neurol, 3(2005).
- H. Foyaca-Sibat, L. Ibanez-Valdes, Insular neurocysticercosis: Our finding and review of the medical literature, Internet J Neurol, 2(2006):31-35.
- L.D. Foyaca-Sibat H Cowan, H. Carabin, G. Serrano-Ocana, R.C. Krecek, A. Willingham, Accuracy of serological exam for the diagnosis of neurocysticercosis in outpatients with epilepsy, Eastern Cape Province, South Africa, PLOS Neglected Trop Dis, 3(2009):1-7.
- H. Foyaca-Sibat, L. Ibanez-Valdes, J. More-Rodriguez, Parasitic zoonoses of the brain: Another challenger?, J Neurol, 12(2009).
- H. Carabin, R. C. Krecek, L. D. Cowan, L. Michael, H. FoyacaâSibat, et al. Estimation of the monetary burden of Taenia solium cysticercosis in the Eastern Cape, South Africa, Trop Med Int Health, 11(2006):906-916.
[Crossref] [Google Scholar] [PubMed]
- H. Foyaca-Sibat, L. Ibanez-Valdes, Treatment of epilepsy secondary to neurocysticercosis. InNovel Treatment of Epilepsy, (2011).
- H. Foyaca-Sibat, Epilepsy secondary to parasitic zoonoses of the brain, InNovel Aspects on Epilepsy, (2011).
- H. Foyaca-Sibat, M. Salazar-Campos, L. Ibanez-Valdes, Cysticercosis of the extraocular muscles. Our experience and review of the medical literature, Internet J Neurol, 14(2012).
- H. Foyaca-Sibat, L. Ibanez-Valdes, Uncommon clinical manifestations of cysticercosis, In Novel Aspects on Cysticercosis and Neurocysticercosis, (2013).
- H. Foyaca-Sibat, L. Ibanez-Valdes, What is a low frequency of the disseminated cysticercosis suggests that neurocysticercosis is going to disappear?, InNovel Aspects on Cysticercosis and Neurocysticercosis, (2013).
- H. F. Sibat, Novel aspects on cysticercosis and neurocysticercosis. BoD Books on Demand. (2013)
- H. Foyaca-Sibat, D.R. Romero, L. Ibanez-Valdes, Prevalence of epilepsy and general knowledge about neurocysticercosis at Ngangelizwe location, South Africa, Internet J Neurol, 4(2005).
- H. FoyacaâSibat, L. F. IbañezâValdés, Subarachnoid cysticercosis and ischemic stroke in epileptic patients, (2018).
- E.V. Noormahomed, F.S.H. Noormahomed, E. Virgínia, N. Nhancupe, J. Mufume, Neurocysticercosis in epileptic children: An overlooked condition in mozambique, challenges in diagnosis, management and research priorities, EC Microbiol, (2021):49.
- H. F. Sibat, Neurocysticercosis, epilepsy, COVIDâ19 and a novel hypothesis: Cases series and systematic review, Clin Schizophr Relat Psychoses, 15S(2021).
- H. F. Sibat, Neurocysticercosis, epilepsy, COVIDâ19 and a novel hypothesis: cases series and systematic review, Clin Schizophr Relat Psychoses, 15S(2021).
- H.F. Sibat, Comorbidity of neurocysticercosis, HIV, cerebellar atrophy and SARS-CoV-2: Case report and systematic review, Clin Schizophr Relat Psychoses, 15(2021).
- L H. Foyaca-Sibat, L. Ibanez-Valdes, Psychogenic nonepileptic seizures in patients living, Seizures, (2018):97.
- D. L. Bridgewater, A case of petrositis, Br Med J, 2(1944):470-471.
[Crossref] [Google Scholar] [PubMed]
- K. R. Ogilvie, Two cases of petrositis and Gradenigo’s syndrome, treated by conservative surgery and penicillin, J Laryngol Otol, 60(1945):445-448.
[Crossref] [Google Scholar] [PubMed]
- S. Horowitz, Gradenigo’s syndrome and report of two cases, J Laryngol Otol, 62(1948):639-647.
[Crossref] [Google Scholar] [PubMed]
- L. G. Eby, Petrositis and lateral sinus thrombosis due to antibioticâresistant infections, Laryngoscope, 71(1961):1165–1185.
[Crossref] [Google Scholar] [PubMed]
- F. D. Gillespie, Gradenigo’s syndrome: Report of a case, Va Med Mon, 89(1962):501-502.
- L. H. Hiranandani, Tuberculous petrositis: A case report, Laryngoscope, 77(1967):1723-1728.
[Crossref] [Google Scholar] [PubMed]
- K. L. Horn, M. D. Erasmus, F. I. Akiya, Suppurative petrous apicitis: Osteitis or osteomyelitis? An imaging case report, Am J Otolaryngol, 17(1996):54–57.
[Crossref] [Google Scholar] [PubMed]
- T. Murakami, J. Tsubaki, Y. Tahara, T. Nagashima, Gradenigo’s syndrome: CT and MRI findings, Pediatr Radiol, 26(1996):684-685.
[Crossref] [Google Scholar] [PubMed]
- A. M. Minotti, S. E. Kountakis, Management of abducens palsy in patients with petrositis, Ann Otol Rhinol Laryngol, 108(1999):897-902.
[Crossref] [Google Scholar] [PubMed]
- P. Jagadeesan, K. Madeswaran, S. P. Thiruppathy, Gradenigo’s syndrome-a rare complication of otitis media, J Indian Med Assoc, 100(2002):669-670.
[Google Scholar] [PubMed]
- K. Piron, F. Gordts, R. Herzeel, Gradenigo syndrome: A caseâreport, Bull Soc Belge Ophtalmol, 290(2003):43-47.
[Google Scholar] [PubMed]
- S. N. Park, S. W. Yeo, B. D. Suh, Cavernous sinus thrombophlebitis secondary to petrous apicitis: A case report, Otolaryngol Head Neck Surg, 128(2003):284-286.
[Crossref] [Google Scholar] [PubMed]
- Y. Finkelstein, N. Marcus, R. Mosseri, Z. Bar-Sever, B. Z. Garty, et al. Streptococcus acidominimus infection in a child causing Gradenigo syndrome, Int J Pediatr Otorhinolaryngol, 67(2003):815-817.
[Crossref] [Google Scholar] [PubMed]
- A. Sethi, A. Sabherwal, A. Gulati, D. Sareen, Primary tuberculous petrositis, Acta Otolaryngol, 125(2005):1236-1239.
[Crossref] [Google Scholar] [PubMed]
- A. M. Visosky, B. Isaacson, J. S. Oghalai, Circumferential petrosectomy for petrous apicitis and cranial base osteomyelitis, Otol Neurotol, 27(2006):1003-1013.
[Crossref] [Google Scholar] [PubMed]
- M. A. Hafidh, I. Keogh, R. M. C. Walsh, M. Walsh, D. Rawluk, et al. Otogenic intracranial complications: A 7âyear retrospective review, Am J Otolaryngol, 27(2006):390-395.
[Crossref] [Google Scholar] [PubMed]
- T.E. Rossor, Y.C. Anderson, N.B. Steventon, L.M. Voss, Conservative management of Gradenigo’s syndrome in a child, BMJ Case Rep, (2011):bcr0320113978.
[Crossref] [Google Scholar] [PubMed]
- T. J. Pollock, P. Kim, M. A. Sargent, M. Aroichane, C. J. Lyons, et al. Ophthalmic complications of otitis media in children, J AAPOS, 15(2011):272-275.
[Crossref] [Google Scholar] [PubMed]
- S.K. Kong, I.W. Lee, E.K. Goh, S.E. Park, Acute otitis mediaâinduced petrous apicitis presenting as the Gradenigo syndrome: Successfully treated by ventilation tube insertion, Am J Otolaryngol, 32(2011):445–447.
[Crossref] [Google Scholar] [PubMed]
- C.L. Jacobsen, M.A. Bruhn, Y. Yavarian, M.L. Gaihede, Mastoiditis and Gradenigo’s syndrome with anaerobic bacteria, BMC Ear Nose Throat Disord, 12(2012):10.
- C. Colpaert, V. Van Rompaey, O. Vanderveken, C. Venstermans, A. Boudewyns, et al. Intracranial complications of acute otitis media and Gradenigo’s syndrome, BâENT, 9(2013):151-156.
[Google Scholar] [PubMed]
- M. Heshin-Bekenstein, O. Megged, U. Peleg, S. Shahroor-Karni, R. Bass, et al. Gradenigo’s syndrome: Is fusobacterium different? Two cases and review of the literature, Int J Pediatr Otorhinolaryngol, 78(2014):166–169.
[Crossref] [Google Scholar] [PubMed]
- P. Y. Chen, C. C. Wu, T. L. Yang, C. J. Hsu, Y. T. Lin, K. N. Lin, et al. Gradenigo syndrome caused by nontuberculous mycobacteria, Audiol Neurootol, 19(2014):275-282.
[Crossref] [Google Scholar] [PubMed]
- K.Y. Choi, S.K. Park, Petrositis with bilateral abducens nerve palsies complicated by acute otitis media, Clin Exp Otorhinolaryngol, 7(2014):59–62.
[Crossref] [Google Scholar] [PubMed]
- Y. Plodpai, S. Hirunpat, W. Kiddee, Gradenigo’s syndrome secondary to chronic otitis media on a background of previous radical mastoidectomy: A case report, J Med Case Rep, 8(2014):217.
[Crossref] [Google Scholar] [PubMed]
- J.M. Valles, R. Fekete, Gradenigo syndrome: Unusual consequence of otitis media, Case Rep Neurol, 6(2014):197-201.
[Crossref] [Google Scholar] [PubMed]
- P.V. Jensen, M.S. Hansen, M.N. Møller, J.P. Saunte, The forgotten syndrome? Four cases of Gradenigo’s syndrome and a review of the literature, Strabismus, 24(2016):21–27.
[Crossref] [Google Scholar] [PubMed]
- T. Kazemi, Acute otitis mediaâinduced Gradenigo syndrome, a dramatic response to intravenous antibiotic, Iran J Otorhinolaryngol, 29(2017):165-169.
- P. V. F. Jensen, M. B. Avnstorp, T. Dzongodza, C. Chidziva, C. von Buchwald, et al. A fatal case of Gradenigo’s syndrome in Zimbabwe and the DanishâZimbabwean ENT collaboration, Int J Pediatr Otorhinolaryngol, 97(2017):181–184.
[Crossref] [Google Scholar] [PubMed]
- M. Vitale, M. Amrit, R. Arora, J. Lata, Gradenigo’s syndrome: A common infection with uncommon consequences, Am J Emerg Med, 35(2017):1388–e1.
[Crossref] [Google Scholar] [PubMed]
- R.A. Dorner, E. Ryan, J.M. Carter, Gradenigo syndrome and cavitary lung lesions in a 5âyearâold with recurrent otitis media, J Pediatric Infect Dis Soc, 6(2017):305-308.
[Crossref] [Google Scholar] [PubMed]
- N. Taklalsingh, F. Falcone, V. Velayudhan, Gradenigo’s syndrome in a patient with chronic suppurative otitis media, petrous apicitis, and meningitis, Am J Case Rep, 18(2017):1039-1043.
[Crossref] [Google Scholar] [PubMed]
- S. Ghani, M. Likeman, M.D. Lyttle, New onset strabismus in association with ear pain, Arch Dis Child Educ Pract Ed, 102(2017):267-269.
[Crossref] [Google Scholar] [PubMed]
- J. Solms, M. Evangelista, A. Gourishankar, A child with right ear pain and a gaze palsy, Clin Pediatr, 56(2017):1072-1074.
[Crossref] [Google Scholar] [PubMed]
- D. Shapiro, D. Vaiyani, D. Horlbeck, S. Pattishall, Case 1: Otorrhea, otalgia, and blurry vision in an 11âyearâold girl, Pediatr Rev, 38(2017):566.
[Crossref] [Google Scholar] [PubMed]
- A. K. Gadre, R. A. Chole, The changing face of petrous apicitis–a 40âyear experience, Laryngoscope, 128(2018):195–201.
[Crossref] [Google Scholar] [PubMed]
- A. BrunetâGarcia, M. V. BarriosâCrispi, M. FaubelâSerra, Carotid canal bone erosion. Gradenigo’s syndrome, Acta Otorrinolaringol Esp, 69(2018):246–247.
[Crossref] [Google Scholar] [PubMed]
- A. AlâJuboori, A. N. Al Hail, Gradenigo’s syndrome and labyrinthitis: Conservative versus surgical treatment, Case Rep Otolaryngol, (2018):6015385.
[Crossref] [Google Scholar] [PubMed]
- N. Bozan, U. Düzenli, A. Yalinkilic, A. Ayral, M. Parlak, et al. Gradenigo syndrome induced by suppurative otitis media, J Craniofac Surg, 29(2018):e645–e646.
[Crossref] [Google Scholar] [PubMed]
- S. Özkaçmaz, Acute otitis media associated with Gradenigo syndrome and transverse sinus thrombosis: A case report, J Int Med Res, 47(2019):1348–1352.
[Crossref] [Google Scholar] [PubMed]
- N. Rossi, M. L. Swonke, L. Reichert, D. Young, Gradenigo’s syndrome in a fourâyearâold patient: A rare diagnosis in the modern antibiotic era, J Laryngol Otol, 133(2019):535–537.
[Crossref] [Google Scholar] [PubMed]
- A. S. Athapathu, E. R. S. Bandara, A. A. H. S. Aruppala, K. M. A. U. Chandrapala, S. Mettananda, A child with Gradenigo syndrome presenting with meningism: A case report, BMC Pediatr, 19(2019):350.
[Crossref] [Google Scholar] [PubMed]
- A. Brambilla, M. Pasti, N. Parri, Sudden diplopia at a pediatric emergency department: A case of Gradenigo syndrome in a child, Pediatr Emerg Care, 35(2019):e236–e237.
[Crossref] [Google Scholar] [PubMed]
- S. Savasta, P. Canzi, F. Aprile, A. Michev, T. Foiadelli, et al. Gradenigo’s syndrome with abscess of the petrous apex in pediatric patients: What is the best treatment?, Childs Nerv Syst, 35(2019):2265–2272.
[Crossref] [Google Scholar] [PubMed]
- J. McLaren, M. S. Cohen, C. M. El Saleeby, How well do we know Gradenigo? A comprehensive literature review and proposal for novel diagnostic categories of Gradenigo’s syndrome, Int J Pediatr Otorhinolaryngol, 132(2020):109942.
[Crossref] [Google Scholar] [PubMed]
- P. D. Patel, A. T. Meybodi, P. Agarwalla, R. W. Jyung, J. K. Liu, Rapid recovery of cranial nerve deficits after anterior petrosal (Kawase) approach for medically refractory petrous apicitis, World Neurosurg, 140(2020):122–127.
[Crossref] [Google Scholar] [PubMed]
- J. Hodges, J. Matsumoto, N. Jaeger, B. Wispelwey, Gradenigo’s syndrome and bacterial meningitis in a patient with a petrous apex cholesterol granuloma, Case Rep Infect Dis, (2020):8822053.
[Crossref] [Google Scholar] [PubMed]
- B. Demir, G. Abuzaid, Z. Ergenc, E. Kepenekli, Delayed diagnosed Gradenigo’s syndrome associated with acute otitis media, SAGE Open Med Case Rep, 8(2020):2050313.
[Crossref] [Google Scholar] [PubMed]
- A. Chandran, P. Sagar, R. Monga, S. Singh, Unusual manifestation of Koch’s disease: GradenigoâLannois syndrome, BMJ Case Rep, 13(2020):e236779.
[Crossref] [Google Scholar] [PubMed]
- G. C. Guimaraes, P. P. de Freitas, V. A. da Silva, A. M. Castilho, Conservative management of petrous apex abscess and Gradenigo’s syndrome in a diabetic patient: Case report and literature review, Clin Case Rep, 9(2021):742–746.
[Crossref] [Google Scholar] [PubMed]
- V. Sattarova, M. Gencturk, M. S. Lee, C. M. McClelland, Gadoxetate disodiumâenhanced imaging of Gradenigo syndrome in endâStage renal disease, J Neuroophthalmol, 41(2021):e375–e377.
[Crossref] [Google Scholar] [PubMed]
- M. Malic, B. Milicic, M. Gjuric, Unrecognized petrous apicitis as a cause of longâlasting headache in a 5âyearâold child: Case report, J Int Adv Otol, 17(2021):468–470.
[Crossref] [Google Scholar] [PubMed]
- Y. Liu, P. K. Yeh, Y. P. Lin, Y. F. Sung, Steroidâresponsive Gradenigo’s syndrome mimicking subdural hematoma, Cureus, 13(2021):e19547.
[Crossref] [Google Scholar] [PubMed]
- J. Quesada, A. Kong, E. Tweddle, An unusual case of acute otitis media resulting in Gradenigo syndrome: CT and MRI findings, Radiol Case Rep, 16(2021):3903–3907.
[Crossref] [Google Scholar] [PubMed]
- L. Bonavia, J. Jackson, Gradenigo syndrome in a 14âyearâold girl as a consequence of otitis media with effusion, J Neuroophthalmol, 42(2022):e408–e409.
[Crossref] [Google Scholar] [PubMed]
- L. Bonavia, J. Jackson, Gradenigo syndrome in a 14âyearâold girl as a consequence of otitis media with effusion, J Neuroophthalmol, 42(2022):e408–e409.
[Crossref] [Google Scholar] [PubMed]
- L. Jin, S. Liu, S. Tan, Petrositis caused by fluconazoleâresistant candida: Case report and literature review, BMC Infect Dis, 22(2022):649.
[Crossref] [Google Scholar] [PubMed]
- K. C. Chan, S. L. Chen, Diplopia in a child: Gradenigo syndrome is an unforgettable disease, Ear Nose Throat J, 102(2023):NP53–55.
[Crossref] [Google Scholar] [PubMed]
- K. Horache, M. Jidal, I. ElOuali, R. Saouab, J. Elfenni, Insights into Gradenigo syndrome: Case presentation and review, Radiol Case Rep, 19(2024):5442–5446.
- Z. Ali, S. Alhantoosh, Z. Albaqal, R. Alkhudaimi, A. S. Alharbi, et al. Gradenigo Syndrome: A Case Report of a Rare Complication of Otitis Media, Cureus, 16(2024):e51865.
[Crossref] [Google Scholar] [PubMed]
- F. Mammarella, A. Loperfido, G. Velletrani, F. Casorati, A. Stasolla, et al. Refractory Pseudomonas Osteomyelitis of the Skull Base with Gradenigo’s Syndrome: Early Dysphagia and Late Abducens Nerve Palsy, J Med Cases, 15(2024):43–48.
[Crossref] [Google Scholar] [PubMed]
- C. Y. Kim, M. Y. Lee, J. Y. Jung, J. E. Choi, Atypical Gradenigo's syndrome in a pediatric case: A critical review of neuroimaging, Radiol Case Rep, 19(2024):2633–2638.
[Crossref] [Google Scholar] [PubMed]
- A. Guennouni, A. S. Houssaini, S. Bahha, M. Fadil, H. EnâNouali, et al. Gradenigo syndrome complicated by a retroâpharyngeal abscess in an 8âyearâold child: A case report and literature review, Radiol Case Rep, 20(2025):1867–1870.
[Crossref] [Google Scholar] [PubMed]
- H. F. Sibat, L. F. I. Valdes, Eradication of neurocysticercosis in South Africa, J Drug Alcohol Res, 14(2025):162719.
- P. Lasjaunias, J. Merland, J. Theron, J. Moret, Dural vascularization of the middle cranial fossa, J Neuroradiol, 4(1977):361–384.
[Google Scholar] [PubMed]
- P. Lasjaunias, J. Moret, J. Mind, The anatomy of the inferolateral trunk (ILT) of the internal carotid artery, Neuroradiology, 13(1977):215–220.
[Crossref] [Google Scholar] [PubMed]
- P. Lasjaunias, J. Brismar, J. Moret, J. Theron, Recurrent cavernous branches of the ophthalmic artery, Acta Radiol, 194(1978):553.
[Crossref] [Google Scholar] [PubMed]
- J. E. MacLennan, A. E. Rosenbaum, V. M. Haughton, Internal carotid origins of the middle meningeal artery, Neuroradiology, 7(1974):265–275.
- A. L. Weber, D. K. Mikulis, Inflammatory disorders of the paraorbital sinuses and their complications, Radiol Clin North Am, 25(1987):615–630.
[Google Scholar] [PubMed]
- M. L. Pensak, The cavernous sinus: An anatomic study with clinical implication, Laryngoscope Investig Otolaryngol, 9(2024):e1226.
[Crossref] [Google Scholar] [PubMed]
- A. Malhotra, L. Tu, V. B. Kalra, X. Wu, A. Mian, et al. Gandhi. Neuroimaging of Meckel’s cave in normal and disease conditions, Insights Imaging, 9(2018):499–510.
[Crossref] [Google Scholar] [PubMed]
- B. K. Tao, F. Alotaibi, A. McAlpine, CeftazidimeâCefazolin Empiric Therapy for Pediatric Gradenigo Syndrome, Ann Otol Rhinol Laryngol, 134(2024):234–237.
[Crossref] [Google Scholar] [PubMed]
- S. Tornabene, G. M. Vilke, Gradenigo's syndrome, J Emerg Med, 38(2010):449–451.
[Crossref] [Google Scholar] [PubMed]
- N. Bozan, U. Düzenli, A. Yalinkilic, A. Ayral, M. Parlak, et al. Gradenigo syndrome induced by suppurative otitis media, J Craniofac Surg, 29(2018):e645–e646.
[Crossref] [Google Scholar] [PubMed]
- G. Talmor, M. Vakil, C. Tseng, P. Svider, M. Ying, et al. Petrous apicitis: A systematic review and case presentation, Otol Neurotol, 43(2022):753–765.
[Crossref] [Google Scholar] [PubMed]
- J. McLaren, M. S. Cohen, C. M. El Saleeby, How well do we know Gradenigo? A comprehensive literature review and proposal for novel diagnostic categories of Gradenigo’s syndrome, Int J Pediatr Otorhinolaryngol, 132(2020):109942.
[Crossref] [Google Scholar] [PubMed]
- B. Demir, G. Abuzaid, Z. Ergenc, E. Kepenekli, Delayed diagnosed Gradenigo’s syndrome associated with acute otitis media, SAGE Open Med Case Rep, 8(2020).
[Crossref] [Google Scholar] [PubMed]
- G. C. Guimaraes, P. P. de Freitas, V. A. R. da Silva, A. M. Castilho, Conservative management of petrous apex abscess and Gradenigo’s syndrome in a diabetic patient: Case report and literature review, Clin Case Rep, 9(2021):742–746.
[Crossref] [Google Scholar] [PubMed]
Copyright: © 2025 Lourdes de Fatima Ibanez Valdes, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.