Research: Journal of Drug and Alcohol Research (2023) Volume 12, Issue 2
Formulation and Evaluation of Herbal Lozenges using Embelia ribes
Sai Datri*, Lakshmana Rao, Mahesh Chandra Narayana, Durga Bhavani, Bhargavi, Yamini Padma Bhavani and MeghanaSai Datri, Department of Pharmaceutical Sciences, V. V. Institute of Pharmaceutical Sciences, India, Email: saidatri@gmail.com
Received: 01-Mar-2023, Manuscript No. JDAR-23-97267 ; Editor assigned: 03-Mar-2023, Pre QC No. JDAR-23-97267 (PQ); Reviewed: 17-Mar-2023, QC No. JDAR-23-97267 ; Revised: 22-Mar-2023, Manuscript No. JDAR-23-97267 (R); Published: 29-Mar-2023, DOI: 10.4303/JDAR/236229
Abstract
Embelin hard candy lozenges were formulated to provide a slow release of the medicament for the treatment of bronchitis. It helps to make breathing easier by means of relaxing and opening air passage in the lungs. The local acting mechanism of embelin makes it more suitable to formulate as lozenges. This research is to develop a new dosage form of embelininto hard candy lozenges and their pharmaceutical standardization. The extraction of embelin from Embelia ribes, formulation of lozenges, processing procedure with extract-based herbal formulation, andexpansion for analytical standardization including qualitative and quantitative determinations were carried out. The formulation of hard candy lozenges was subjected to physico-chemical as well as in vitro drug release. Among all the formulations of hard candy lozenges F10 had shown in vitro drug release of 100.6% at the end of 30 minutes.
Keywords
Embelin; Bronchitis; Lozenges; Extraction
Introduction
Lozenges are flavored medicated dosage forms intended to be dissolved or disintegrated in the mouth or pharynx containing one or more medicaments usually in the sweetened base. Lozenges are used for those patients who cannot swallow solid dosage forms as well as for medications designed to be released gradually to yield a constant level of drug to bath the throat tissues by the solution of the drug in the oral cavity. Lozenges, which may consist of one or more pharmaceuticals in the carrier, have been used to deliver medications, either topically within the buccal cavity of the patient or by swallowing the pharmaceutical after it has dissolved in saliva [1]. Herbal lozenges, also known as herbal pastilles or herbal tablets, are a method of consuming an herb without needing to prepare a portion of food or beverage in which to incorporate it [2].
Bronchitis is an inflammation of the lining of bronchial tubes, which carry air to and from lungs [3]. People who have bronchitis frequently cough up thickened mucus that can be discolored. The medicinal woody climber Embelia ribes Burm F belongs to the Myrsinaceae family [4]. Embelia ribes contains embelin, which has bronchitis and sore throat properties
Materials and Methods
Collection of herbs
Herbs samples were purchased from local Ayurvedic store in Gudiwada, storage conditions were properly maintained with respect to light and temperature. The herbs were recognized and compared with authentic specimen by taxonomist available at the Krishna University Herbarium (KUH). Similarly, it was also made sure that every other material including solvents, reagents and chemicals must be of pure analytical grade and acquired with proper documentation of COA’s and MSDS forms through local supplier.
Preparation of extracts
The fruits of Embelia ribes Burm F. were condensed to a powder of 40# and stored in an amber colored bottle till further use. The powdered drug was extracted by methanol using Maceration for about 24 h and the embelin was isolated [5].
Determination of melting point of embelin
The electrical melting point apparatus using capillary method measured the melting point of drug.
Determination of λ max of embelin
Embelin solution in methanol was prepared, then the solution was scanned by spectrophotometer from 400 nm-800 nm, and λ max of the drug was determined.
Drug-excipient compatibility studies
Compatibility study was carried out by recording the sample using Fourier Transform Infra-Red Spectrophotometer (FTIR) in the range of 4000 cm-1 to 400 cm-1 by Potassium Bromide pellet technique.
Preparation of calibration curve for embelin
100 mg of Embelin was accurately weighed and is dissolved in methanol and the volume was made up to 100 ml to obtain a stock solution of 1000 μg/ml, 0.2 ml, 0.4 ml, 0.6 ml, 0.8 ml, 1.0 ml and 1.21 ml of this stock solution is again diluted with methanol up to 10 ml to obtain a solution with concentrations of 20 μg/ml, 40 μg/ml, 60 μg/ml, 80 μg/ml, 100 μg/ml and 120 μg/ml. The resulting solution is scanned at 294.3 nm in a double beam UV-Visible spectrophotometer.
Preparation of embelin medicated hard candy lozenges
The lozenges were formulated by heating and congealing technique. Syrupy base was prepared in a beaker by dissolving the required amounts of sugar in water and kept for heating on a hotplate. Temperature was maintained at 105°C-110°C till it became thick. The drug and other excipients (except plasticizer) were added manually and mixed thoroughly after 30 minutes with continue process of heating. The prepared mass was further heated for 45 minutes and then plasticizer was added into it. Then above syrupy base was poured into pre-cooled and pre lubricated mould and the mould was kept aside for 10-15 minutes. Prepared lozenges were separated from mould and were kept for air drying.
Evaluation parameters
Physical parameters: The medicated lozenges were examined in terms of clarity, texture and consistency. Texture of lozenges in terms of stickiness was evaluated by visual inspection of the product [6-22].
Weight variation test: 10 lozenges from each batch were individually weighed in grams on an analytical balance. The average weight and standard deviations are measured.
Thickness test: The thickness in millimetres was measured individually for 10 pre weighed lozenges by using verniercalipers.
Hardness test: The hardness of lozenges was measured using a Monsanto Hardness Tester.
Drug content uniformity: The content uniformity was tested by measuring weight equivalent to one lozenge and dissolving the powder content in 100 ml volumetric flask containing 50 ml of 6.8 phosphate buffers and stands it for 30 minutes. The mixture was made up to volume with buffer pH 6.8. The diluted samples absorption was recorded at 294.3 nm.
Moisture content: By Gravimetric method, one gram sample is weighed and placed in a desiccator at for 24 h.
Mouth dissolving time: The time taken by the lozenge to dissolve completely was determined by placing each lozenge in separate beaker containing 100 ml phosphate buffer pH 6.8 at 50 rpm using mechanical stirrer and time was noted at 37°C.
In-vitro drug release: The modified dissolution test apparatus USP type II (paddle) is used and 250 ml of the dissolution medium phosphate buffer pH 6.8 is placed in the beaker containing the lozenge and stirred at 100 rpm. 5 ml aliquot samples are withdrawn at 5 minutes interval and replaced immediately with an equal volume of fresh medium i.e., phosphate buffer pH 6.8. Each aliquot is diluted and analyzed using blank, by UV-Visible spectrophotometer. The amount of drug release is determined from the standard calibration curve of pure drug.
In-vitro release kinetics of optimized formulation: To study the kinetics, data obtained from in-vitro release were plotted in various kinetic models.
Stability studies: The optimized formulations were subjected to stability studies at temperature i.e., 40ºC/75% RH for a period of month.
Results and Discussion
The primary goal of this study was to formulate and evaluate embelin lozenges for patients suffering from bronchitis. Embelin initially was tested for the melting point and FTIR as an identification tests. The melting point of embelin sample was found to be 142.00°C ± 1°C which is as per the specifications mentioned in IP and FT-IR plot is shown in Figure 1. Absorption maxima for embelin when observed under UV–visible spectrophotometer were found to be at 294.3 nm. This was observed from the peak in Figure 2. Calibration curve of embelin was obtained at concentration of 20 μg/ml-120 μg/ml with R2 value=0.999. Embelin lozenges were prepared using different ingredients by heating and congealing technique. Around 10 formulations were prepared with a total weight of 3000 mg. Various ingredients such as sucrose, detrose, mannitol, inverted sugar, glycerine, xanthan gum, guar gum, vegetative oil, lemon juice and cherry flavor were included in various proportion. Sucrose, detrose, mannitol, and inverted sugar were used as hard candy bases, xanthan gum and guar gum as a whipping agent, glycerine was selected as a plastisizer. Lemon juice was used as anti-oxident. Vegetable oil was used as a lubricant. Cherry flavor was utilized as a flavoring agent and colouring agent. The composition of lozenges using these excipients is shown in Table 1. Photographs of prepared lozenges are shown in Figure 3.
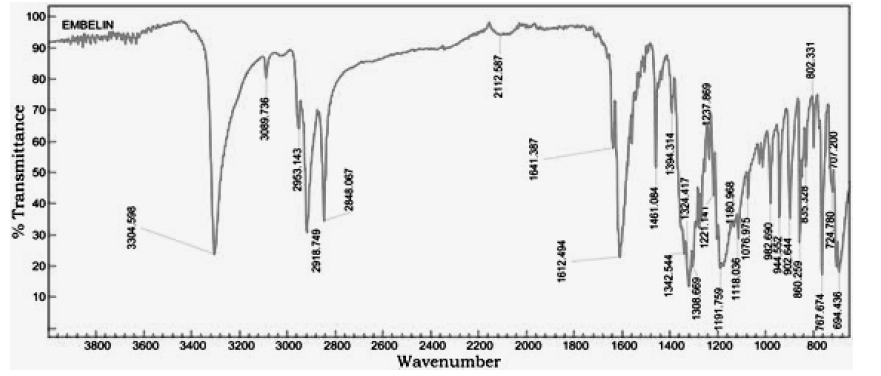
Figure 1: FT-IR spectrum of Embelin
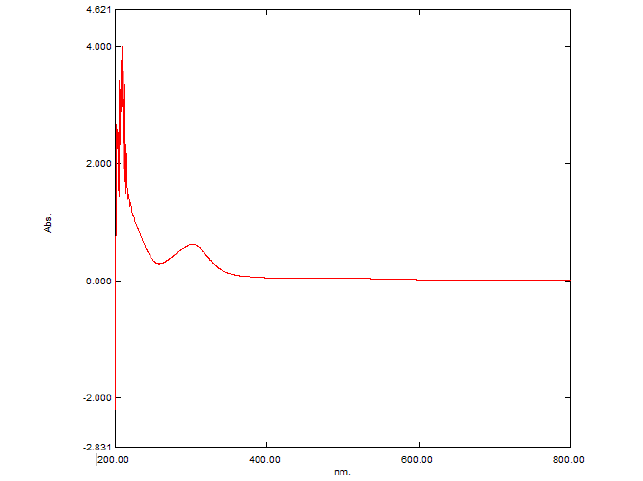
Figure 2: UV spectrum of Embelin in Methanol

Figure 3: Photographs of prepared lozenges
| Ingredients | Composition per lozenges | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | |
| Embelin (mg) Active Ingredient |
5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Mannitol | 18.8 | - | - | - | - | - | - | - | - | - |
| Dextrose | - | 18.8 | - | - | - | - | - | - | - | - |
| Sucrose | - | - | 18.8 | - | - | - | - | - | - | - |
| Inverted Sugar | - | - | - | 48.4 | 48.4 | 48.4 | 48.4 | 48.2 | 48.6 | 48.0 |
| Glycerine (ml) | - | - | - | - | 0.5 | 0.75 | 1 | 1 | 1 | 1 |
| Xanthan Gum | - | - | 0.075 | 0.075 | 0.075 | 0.075 | 0.075 | 0.070 | 0.05 | 0.025 |
| Guar Gum | 0.075 | 0.075 | - | - | - | - | - | - | - | - |
| Distilled Water | 25.26 | 25.26 | 25.26 | 25.26 | 25.26 | 25.26 | 25.26 | 25.26 | 25.26 | 25.26 |
| Lemon Juice | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. |
| Cherry Flavor | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. |
Table 1: Formulation table for Embelin medicated hard candy lozenges
The physical compatibility was observed visually. The study reveals that the drug and the excipients were physically compatible with each other as there was no change of colour. The chemical compatibility studies are performed by FT-IR Studies, there is no change in major peaks reveals that the excipients are compatible with the drug selected for the formulation. In physical appearance evaluation, all the formulations were translucent and smooth in nature. F1, F5, and F6 formulations show slightly thick and sticky in nature. All other formulations were good and acceptable. The formulated lozenges were subjected to different evaluation tests. The results of post-formulation parameters of lozenges such as weight variation, hardness, thickness, and Mouth dissolving time are shown in Table 2. The results of moisture content and drug content are shown in Table 3. All the values of the parameters in the hard candies were observed within the limits with no significant deviation. As all the materials were free flowing, lozenges formulated were of same weight due to uniform mold fill. The developed hardness range shows good mechanical strength with an ability to withstand physical and mechanical stress conditions. Percentage of drug content was calculated for all the formulations. The drug content for all the formulations was found to be within the range as per the specifications of IP 2007. In vitro release studies were conducted for all the formulations. Percentage drug release from lozenges is shown in Table 4 and the release profile is represented in Figures 4-6. The cumulative percentage of drug release for the F1 which contains the mannitol as sugar showed 99.4% at 15 minutes. F2, F3 formulations containing dextrose and sucrose as a base showed the drug release 92.4%, 96.8% respectively within 10 minutes. In F4 formulation Inverted Sugar as a sugar base it showed the drug release 101% at 15 minutes. The addition of plasticizers in F5, F6, F7 formulations showed the increase in time for the drug release. F5 formulation showed the release rate at 100.4% within 20 minutes. For F6 cumulative drug release 97.7% at 15 minutes and for F7 99.6% in 25 minutes. The formulations F8, F9, F10 contains 0.75%, 0.5%, 0.25% of xanthan gum as polymer showed the cumulative drug release of Embelin 95.02%, 98.6%, 100.6% respectively in 30 minutes. Hence the formulation F10 containing 0.25% xanthan gum which showed 100.6% drug release at the end of 30 minutes was considered as the optimized formulation.
| S. No. | F Code | Weight of lozenges | Hardness of lozenges (Kg/cm2) | Thickness of lozenges (mm) | Mouth dissolving time (min) |
|---|---|---|---|---|---|
| 1 | F1 | 2.5035 ± 0.032 | 10.5 ± 0.57 | 12.25 ± 0.051 | 10.16 ± 0.51 |
| 2 | F2 | 2.493 ± 0.039 | 10.75 ± 0.92 | 12.23 ± 0.044 | 11.00 ± 0.3 |
| 3 | F3 | 2.488 ± 0.059 | 7.3 ± 0.42 | 12.02 ± 0.043 | 7.06 ± 0.43 |
| 4 | F4 | 2.515 ± 0.023 | 13.6 ± 0.64 | 12.24 ± 0.054 | 12:50 ± 0.19 |
| 5 | F5 | 2.507 ± 0.030 | 10.65 ± 0.57 | 12.24 ± 0.057 | 15:61 ± 0.45 |
| 6 | F6 | 2.508 ± 0.036 | 9.65 ± 0.56 | 12.25 ± 0.054 | 13.47 ± 1.25 |
| 7 | F7 | 2.518 ± 0.041 | 15.25 ± 0.63 | 12.26 ± 0.054 | 16:65 ± 0.36 |
| 8 | F8 | 2.511 ± 0.038 | 15.45 ± 0.43 | 12.25 ± 0.054 | 24:20 ± 0.56 |
| 9 | F9 | 2.519 ± 0.040 | 15.50 ± 0.74 | 12.24 ± 0.041 | 29:05 ± 0.31 |
| 10 | F10 | 2.503 ± 0.029 | 15.30 ± 0.74 | 12.24 ± 0.054 | 30:28 ± 0.75 |
Table 2: Evaluation of Embelin lozenges for post-formulation parameters
| S. No. | F Code | Moisture content of lozenges (%) | Drug content (%) |
|---|---|---|---|
| 1 | F1 | 1.19 | 93.13 ± 2.32 |
| 2 | F2 | 1.44 | 91.43 ± 3.31 |
| 3 | F3 | 2.10 | 94.84 ± 3.85 |
| 4 | F4 | 1.02 | 98.24 ± 3.5 |
| 5 | F5 | 1.15 | 90.22 ± 2.50 |
| 6 | F6 | 0.94 | 95.15 ± 2.65 |
| 7 | F7 | 0.69 | 92.38 ± 3.52 |
| 8 | F8 | 0.93 | 95.29 ± 2.53 |
| 9 | F9 | 0.84 | 97.60 ± 2.96 |
| 10 | F10 | 0.83 | 99.68 ± 1.50 |
Table 3: Evaluation of Embelin lozenges for post-formulation parameters
| Time (min.) | Percentage Drug Release (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 53 | 73.2 | 84.5 | 71.5 | 51.5 | 63 | 23 | 23 | 31.4 | 25.5 |
| 10 | 74.2 | 92.4 | 96.38 | 96.86 | 88.06 | 82.02 | 41.92 | 33.8 | 40.2 | 38.4 |
| 15 | 99.4 | - | - | 101.1 | 96.5 | 97.7 | 79.4 | 51.48 | 62.2 | 71 |
| 20 | - | - | - | - | 100.14 | - | 92.14 | 80.6 | 82 | 83.6 |
| 25 | - | - | - | - | - | - | 99.6 | 88.6 | 94.2 | 91.4 |
| 30 | - | - | - | - | - | - | - | 95.02 | 98.6 | 100.6 |
Table 4: In-vitro drug release study of medicated lozenges
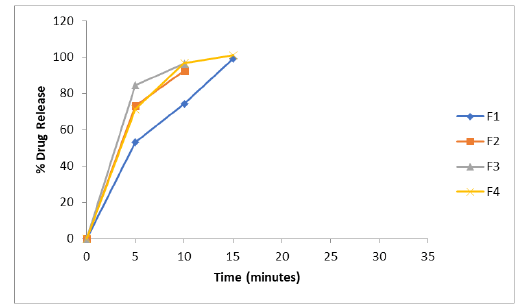
Figure 4: In-vitro release profile of the formulations F1 to F4 containing varying sugar bases
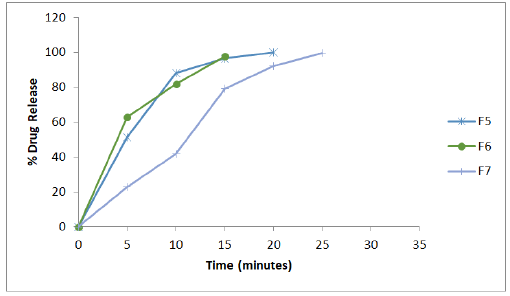
Figure 5: In-vitro release profile of the formulations F5 to F7 containing varying concentrations of plasticizer
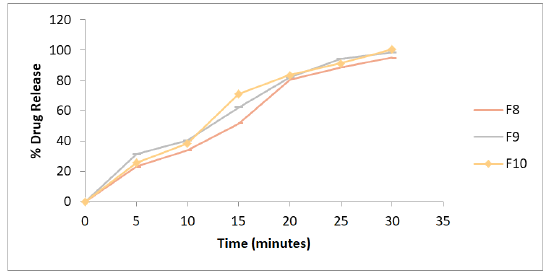
Figure 6: In-vitro release profile of the formulations F8 to F10 containing varying concentrations of xanthan gum
All formulations drug release kinetics results revealed that the mode of drug release from lozenges followed the zero- order release kinetics with R2>0.956 (Table 5). Data were also united into the Korsmeyer–Peppas equation to measure the drug release mechanism further. The value n lies in between 0.69 and 0.147 which indicated that non-Fickian diffusion (anomalous diffusion), the process was significant. Based on the results of post-formulation parameters and in vitro drug release, formulation F10 was considered as an optimized formulation. Additionally, stability studies were also performed for the formulation. Aging studies were performed at conditions of 40°C ± 2°C and 75% RH ± 5% for a period of 1 month. The acquired results are shown in Table 6. From the above data, it was found that there was insignificant change in the release characteristics and physicochemical properties of the lozenges used in the study. Based on the results, it can be concluded that the formulated lozenges were stable at stability conditions (40°C ± 2°C and 75% ± 5% RH) over a period of 1 month. Even though its stability was assured for 1-month, further studies as per the ICH guidelines are needed to establish its shelf-life.
| Time in mins | Square root of time | Log Time | % Cum. Drug Release | % Cum. Drug Remaining | Log % Cum. Drug remaining | Log % Cum. Drug Release | Cube root of % drug remaining |
|---|---|---|---|---|---|---|---|
| 0 | 0 | ∞ | 0 | 100 | 2 | ∞ | 4.64159 |
| 5 | 2.23607 | 0.69897 | 25.5 | 74.5 | 1.87216 | 1.40654 | 4.20777 |
| 10 | 3.16228 | 1 | 38.4 | 61.6 | 1.78958 | 1.58433 | 3.94936 |
| 15 | 3.87298 | 1.17609 | 71 | 29 | 1.4624 | 1.85126 | 3.07232 |
| 20 | 4.47214 | 1.30103 | 83.6 | 16.4 | 1.21484 | 1.92221 | 2.54067 |
| 25 | 5 | 1.39794 | 91.4 | 8.6 | 0.9345 | 1.96095 | 2.0488 |
| 30 | 5.4772 | 1.47712 | 100.6 | -0.6 | - | 2.0026 | -0.8434 |
Table 5: In-vitro release kinetics of optimized formulation
| Evaluation Parameter | Optimized Formulation (F10) | After Stability Study of 1 Month |
|---|---|---|
| Weight variation | 2.503 ± 0.0290 | 2.507 ± 0.030 |
| Hardness | 15.30 ± 0.74 | 15 ± 0.08 |
| Thickness | 12.26 ± 0.054 | 12.18 ± 0.16 |
| Moisture Content | 0.83% | 0.69% |
| Mouth Dissolving Time | 30:28 ± 0.75 | 29:61 ± 0.98 |
| Content uniformity | 99.68% ± 1.50 | 96.48 ± 1.20 |
| Drug release | 100.6% | 99.2% |
Table 6: Evaluation parameters after the stability studies
Conclusion
The physical and chemical compatibility study showed that the drug and excipients are physically compatible with each other. After performing the formulation of batches for sugar selection (F1 to F4). Mannitol was easily dissolved in water, but the mass formulated with mannitol was stuck on the wall of the beaker while heating and a thick viscous mass was not obtained. The batches formulated with dextrose and sucrose showed re-crystallization of sugars. A batch formulated with inverted sugar showed hard candy-type lozenges, but the appearance was not good. A plasticizer like glycerine was added to improvise the appearance. All formulations were prepared and evaluated for in-vitro drug release, physical appearance, weight variation, thickness, hardness, moisture content, mouth dissolving time, and drug content and formulation F10 is satisfied all the parameters and selected as an optimized formulation. The stability studies were performed and there was no change in drug content, friability, and weight variation. Hence the optimized lozenges pass all the parameters and were found to be more effective in the treatment of bronchitis. Hence this formulation can be recommended for patients.
Acknowledgement
The authors are grateful to V. V. Institute of Pharmaceutical Science, Gudlavalleru for providing the necessary facilities to carry out the research work.
Conflict of Interest
None.
References
- M. Rathod, S. Poharkar, Y. Pandhre, M. Muneshwar, S. Sul, A review on medicated lozenges as an easy to use dosage form, Wld J Pharm Res, 7(2018):305-322.
- K. Payal, H. Uditi, N. Kajal, D. Shivani, G. Kumar, Effectiveness of herbal tinctures formulated into lozenges: A current stipulation in paediatrics patients, Int Erl Chldhd Spl Edu, 14(2022):1913-1919.
- W.J. Hueston, A.G. Mainous, Acute bronchitis, Am Fam Phys, 57(1998):1270-1276.
- R. Sandeep, S. Gyanranjan, P. Deepayan, M. Udit Nandan, P. Ajay Kumar, et al. (Vai vidanga)-an overview, Int J of Mdrn Agr, 10(2021):4588-4594.
- K. Pundarikakshudu, H. Joshi, P. Shah, S. Panchal, A simple, facile method for isolation of embelin from fruits of Embelta ribes Burm.f., Indn Drg, 53(2016):23-27.
- H. Shilpa, S. Roma, S. Amruta, T. Sonia, Formulation and development of poly-herbal lozenges: A solace for mouth ulcer, J Med Pharm Alld Sci, 1(2022):166-169.
- A. Altanbat, S. Erdenechimeg, L.T. Serendulam, U.L. Khamsuren, Technological study of lozenges, J Sapp Med, 55(2021).
[Crossref] [Google Scholar] [PubMed]
- A.P. Chaithanya, D. Prothibha, T. Ajith Babu, K.K. Adila, S. Ansiya, Formulation and evaluation of herbal lozenges for pharyngitis, Wrd J Pharm Res, 9(2020):1400-1409.
- A. Amruta, A. Joshi, M. Gaurav, A. Mahesh kumar, A. Utkarsha, Formulation and evaluation of anti-emetic H. spicatum lozenges, J Emer Tech Innv Res, 7(2020):771-776.
- A. Kumar, M. Kumar, K.S. Chandrashekar, P. Girish, P. Vasudev, Development and evaluation of polyherbal lozenges for cold and flu, Ind J Pharm Edu Res, 53(2019):S159-S163.
- D.P. Apurva, Shrikant, K. Tilloo, M.M. Bodhankar, A review on medicated chewable lozenges, Int J Rec Sci Res, 10(2019):32071-32076.
- S. Hanny, W. Setyan, Formulation of chewable lozenges of somjawa (Talinum paniculatum (Jacq.) Gaertn) leaves extract applied for Candida albicans topical infection, Idnsn J Med Hlth, 10(2019):14-23.
- D.M. Kannur, S.S. Salunkhe, P.S. Godbole, S.P. Patil, Formulation and evaluation of traditional medicine based herbal lozenges, jellies and dispersible tablets, Int J Pharm Sci Res, 9(2018):3501-3505.
- A. Binu, I. Thomas, P. Beena, Abrahaformulation and evaluation of herbal lozenges containing eucalyptus oil and coleus aromaticus oil, Amc J Pharm Tech Res, 8(2018):316-322.
- M. Kamlendra Kumar, T. Kutubuddin, J. Vikas, S.C. Mahajan, Formulation and evaluation of herbal lozenges, J Drg Del Thrps, 7(2017):87D-89D.
- R. Hina, Z. Aqib, S.K.Z. Ahmed, N. Safila, U. Khan, Polyherbal extract based linkus lozenges for symptomatic relief: Design, development and evaluation, Amc J Adv Drug Del, 5(2017):011-018.
- K. Doris, O. Kwabena, Dosage forms of herbal medicinal products and their stability considerations-an overview, J Crit Rev, 4(2017):1-8.
- B. Elmira, M.D. Kamali-nejad, S.M. Foroutan, Formulation and physicochemical evaluation of lozenge tablets containing Salvia officinalis, J Yng Pharm, 6(2014):34-38k.
- P. Renuka, Y. Madhusudan, Lozenges formulation and evalution, a current stipulation in paediatrics a review, Int J Adv Pharm Res, 5(2014):290–298.
- Y. Shobhnat, Galib, P.K. Prajapati, Pharmaceutical standardization of herbal lozenges vasa candy, Ayurpharm, Int J Ayur Alld Sci, 3(2014):22–27.
- B. Niko, K. Bistra, N.R Irina, Dimitar, Development and evaluation of novel lozenges containing marshmallow root extract, Pak J Pharm Sci, 26(2013):1103-1107.
- C.O. Esimone, P.U. Onuh, N.C. Obitte, E.M. Egege, K.C. Ugoeze, In vitro evaluation of lozenges containing extracts of roots of Zapotecaportoricensis, J Pharm Tox, 2008:1-6.
Copyright: © 2023 Sai Datri, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

