Research: Journal of Drug and Alcohol Research (2023) Volume 12, Issue 1
Exploring the Antibacterial Activities and Preliminary Sensing Studies of a Quinoline-Functionalized Thiosemicarbazone Derivative
Waseem Shoukat1,2,3, Mazhar Hussain1, Natasha Mukhtiar4, Shujaat Hussain1, Muhammad Nadeem Shoukat1, Muhammad Ghazanfar Bashir5 and Fazal Ur Rehman M.6,7,82Department of Biochemistry, Bahauddin Zakariya University, Multan-60800, Pakistan
3Institute of Pure & Applied Biology, Bahauddin Zakariya University, Multan-60800, Pakistan
4Institute of Biomedical Engineering and Technology, LUMHS, Jamshoro, Pakistan
5Department of Pharmacology, Riphah International University, Lahore-54000,, Pakistan
6Department of Chemistry, Lahore Garrison University, Lahore, Pakistan
7Department of Chemistry, University of Education Lahore, Pakistan
8Advance Analytical Development Lab-Research and Development Department, CCL Pharmaceuticals, Lahore-54000, Pakistan
Waseem Shoukat, Institute of Pure & Applied Biology, Bahauddin Zakariya University, Multan-60800, Pakistan, Email: merawaseemshoukat@gmail.com
Received: 31-Jan-2023, Manuscript No. JDAR-23-92214 ; Editor assigned: 02-Feb-2023, Pre QC No. JDAR-23-92214 (PQ); Reviewed: 16-Feb-2023, QC No. JDAR-23-92214 ; Revised: 21-Feb-2023, Manuscript No. JDAR-23-92214 (R); Published: 28-Feb-2023, DOI: 10.4303/JDAR/236224
Abstract
This research paper describes the synthesis and characterization of a novel Thiosemicarbazone derivative 1 with a quinoline moiety functionalized to it. Spectroscopic techniques, including 1H and 13C NMR, UV-vis absorption, and fluorescence, were used to characterize the synthesized product. The compound was prepared as a solution in ACN and was subjected to various ions. The addition of different ions resulted in changes in color and fluorescence intensity of the compound. The antibacterial activity of the Thiosemicarbazone derivative was also evaluated against gram-positive and gram-negative bacteria using the Kirby-Bauer disc diffusion method. The compound was found to be moderately active against the tested bacterial strains, which could be attributed to the presence of the imine group (-C=N) in the compound. This study presents the first report of the antibacterial activity of benzylmethyl-4-methyl-3-thiosemicarbazone on bacteria. Further research on the compound’s activity against other pathogens, such as malaria and onchocerciasis, is recommended.
Keywords
Thiosemicarbazone; Fluorimetric; Chemosensor; Quinoline
Introduction
Thiosemicarbazones are a class of organic compounds that contain a thiosemicarbazide functional group (R1R2NN= C=S) and a carbonyl group (C=O) that are connected through a methylene (CH2) or ethylene (CH2CH2) bridge. They are versatile molecules with a wide range of biological activities, including antimicrobial, antiviral, anticancer, and ion sensing properties [1,2]. In this article, we will focus on the antimicrobial and ion sensing properties of Thiosemicarbazones. Thiosemicarbazones have been shown to exhibit significant antimicrobial activity against various strains of microorganisms, making them promising candidates for the development of new antimicrobial agents. One of the primary mechanisms of antimicrobial activity of Thiosemicarbazones is believed to be through inhibition of bacterial enzymes, such as thymidylate synthase, dihydrofolate reductase, and ribonucleotide reductase, which are involved in DNA synthesis and replication. Thiosemicarbazones have also been found to disrupt bacterial cell membranes, leading to cell death [3].
Thiosemicarbazones have been investigated for their antimicrobial activity against a wide range of bacteria, including both Gram-positive and Gram-negative strains [4-6]. For example, Thiosemicarbazones have been found to be effective against Staphylococcus aureus, a common bacterial pathogen that can cause skin infections, pneumonia, and sepsis. Thiosemicarbazones have also been shown to be effective against Escherichia coli, a Gram-negative bacterium that can cause urinary tract infections, pneumonia, and sepsis [7].
In addition to their antibacterial activity, Thiosemicarbazones have also been investigated for their antifungal and antiviral activity [8]. Thiosemicarbazones have been shown to be effective against various strains of fungi, including Candida albicans, which can cause a range of infections, including oral thrush and vaginal yeast infections [9]. Thiosemicarbazones have also been shown to be effective against a number of viruses, including herpes simplex virus type 1 (HSV-1) and influenza virus [10].
Ion sensing is the detection of the presence of ions in a sample, typically through changes in a physical or chemical property of the sensing material [11,12]. Thiosemicarbazones have been investigated for their ability to act as ion sensors, particularly for metal ions [13]. One of the most promising applications of Thiosemicarbazones as ion sensors is in the detection of metal ions in environmental samples, such as water and soil. Metal ions can be harmful to the environment and to human health if present in high concentrations, so it is important to be able to detect them accurately and efficiently [14]. Thiosemicarbazones have been shown to be effective sensors for a wide range of metal ions, including copper, nickel, zinc, and mercury. Thiosemicarbazones can act as ion sensors through a variety of mechanisms [15]. For example, some thiosemicarbazones can form complexes with metal ions, which can lead to changes in their fluorescence properties, making them useful for fluorescent sensing. Other Thiosemicarbazones can undergo changes in their electrochemical properties in the presence of metal ions, making them useful for electrochemical sensing.
Methods and Materials
The solvents from Sigma-Aldrich, Acros, and Fluka were used as received, as well as the ion salts, which were in the form of tetrabutylammonium salts for anions and perchlorate for cations, with the exception of Cu(I), Pd(II), and Li(I), which were in the form of tetrafluoroborate and Sn(II), in the form of chloride. On silica gel 60 plates, thin layer chromatography (TLC) was carried out using the fluorescence indicator F254 (Macherey-Nagel). Using the solvent peak as an internal reference, the 1H and 13C nuclear magnetic resonance spectra were captured using a Bruker Avance III apparatus at 400 MHz and 100.6 MHz, respectively. Using two-dimensional heteronuclear correlation methods and Sigma-DMSO-d6 Aldrich’s with a 99.9% deuteration degree and 0.1% v/v tetramethylsilane as the solvent, the task of the 1H and 13C signals was carried out. Using UV-grade solvents and conventional 10 mm optical path quartz cells, the UV-visible absorption and fluorescence spectra were collected using a Shimadzu UV/2501PC spectrophotometer and a Horiba Jobin-Yvon FluoroMax-4 spectrofluorometer, respectively.
Synthesis and spectroscopic characterization of thiosemicarbazone derivative 1
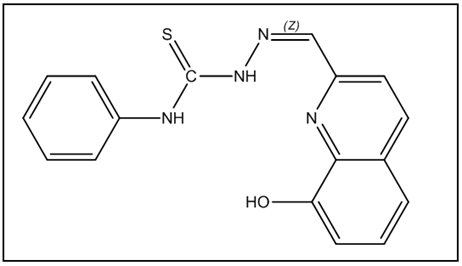
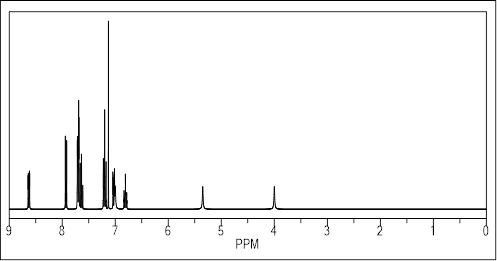
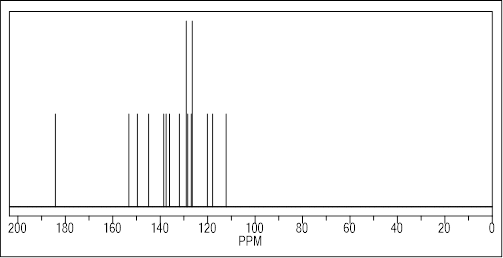
8-hydroxy-quinoline-2-carbaldehyde (0.4 mmol) and 4-phenyl-3-thiosemicarbazide (0.4 mmol) were dissolved in methanol (30 mL). For 70 hours, the reaction mixture was kept dark and stirred at room temperature. TLC was used to monitor the reaction, and DCM/MeOH (50:1) was used as the eluent. Following the evaporation of the solvent at decreased pressure, compound 1 (Figure 1) was produced. By using 1H and 13C NMR spectroscopy, the NH group’s typical signals of 10.35 and 12.19 ppm and the imine CH’s characteristic sign of 8.35 ppm were used to describe the Thiosemicarbazone 1 (Figures 2 and 3).
Figure 1: Chemical structure of prepared Thiosemicarbazone 1.
Figure 2: Predicted 1H-NMR Spectra of prepared Thiosemicarbazone 1.
Figure 3: Predicted 13C-NMR Spectra of prepared Thiosemicarbazone 1.
1H NMR (DMSO-d6, 400 MHz): d=7.09 (dd, J=1.6 and 7.6 Hz, 1H, H7’), 7.23 (dt, J=0.8 and 7.6 Hz, 1H, H4), 7.41- 7.36 (m, 3H, H3+H5+H50), 7.43 (t, J=8.0 Hz, 1H, H60), 7.53 (dd, J=1.2 and 7.6 Hz, 2H, H2+H6), 8.28 (d, J=8.8 Hz, 1H, H40), 8.35 (s, 1H, N=CH), 8.54 (d, J=8.4 Hz, 1H, H30), 9.91 (s, 1H, OH), 10.35 (s, 1H, NH-C=S), 12.19 (s, 1H, NH-N) ppm.
13C NMR (DMSO-d6, 100.6 MHz): d=112.32 (C70), 118.02 (C50), 118.92 (C30), 125.91 (C4), 126.49 (C2+C6), 128.37 (C3+C5), 128.42 (C60), 129.05 (C4a0), 136.35 (C40), 138.37 (C8a0), 139.10 (C1), 143.23 (N=CH), 151.82 (C20), 153.54 (C8), 176.75 (C=S) ppm.
Photophysical characterization of thiosemicarbazone 1
In order to get the data needed to assess the material’s potential as a colorimetric and/or fluorimetric ion sensor; this characterization was carried out to assess the absorption and emission characteristics. UV-vis absorption and fluorescence spectroscopies were used to examine solutions of chemical 1 in ACN at concentrations of 1×10-5 and 1×10-6 M, respectively. The fluorescence standard 9, 10-diphenylanthracene (DPA) in ethanol, whose absolute fluorescence quantum yield is 0.95, was used to compute the compound’s relative quantum fluorescence yield.
Preliminary chemosensing studies of thiosemicarbazone 1 in ACN
Fluorescent chemosensors have become an increasingly popular research topic in the field of analytical chemistry, owing to their high sensitivity and selectivity in detecting various analytes. Thiosemicarbazone derivatives have been widely studied for their potential as chemosensors due to their ability to bind with metal ions and their fluorescence properties. Quinoline, a nitrogen-containing heterocyclic compound, has also been used as a fluorescent probe due to its favorable photophysical properties. Ions and the synthesised product were prepared as solutions in ACN at concentrations of 1×10-5 and 1×10-6 M, respectively. Then, 1 mL of Thiosemicarbazone 1 solution was added along with 50 equivalents of each ion. A UV-vis chamber with 365 nm ultraviolet light and “naked eye” examination of the colour and fluorescence changes were both used.
Antimicrobial activity
Antimicrobial resistance has become a major public health challenge in recent years, and the development of new antibacterial agents is of utmost importance. Quinoline-functionalized Thiosemicarbazone derivatives have shown potential as antimicrobial agents, and have been the focus of increasing attention in drug discovery research. Thiosemicarbazones are well-known for their ability to chelate metal ions and their potential as antimicrobial agents. Quinoline, on the other hand, is a heterocyclic compound that has been extensively used in medicinal chemistry due to its broad spectrum of biological activities, including antibacterial, antiviral, and antimalarial activities. The antibacterial activity of the Thiosemicarbazone Derivative 1 was determined using a modified Kirby-Bauer disc diffusion method [15]. The antibacterial activity was done by using gram +ve organisms: Staphylococcus epidermidis and Bacillus cereus as well as gram –ve organisms: Escherichi coli and Klebsiella pneumoniae. Ciprofloxacin was used as the standard. The percent activity index for the antibacterial was calculated as reported in literature [14].
Results and Discussion
Synthesis and spectroscopic characterization of thiosemicarbazone derivative 1
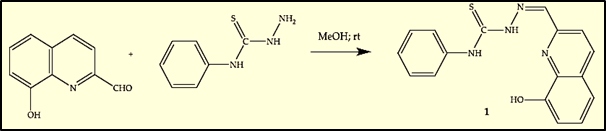
The synthesis of Thiosemicarbazone derivative 1 was performed from commercially available 8-hydroxy-quinoline- 2-carbaldehyde (I) and 4-phenyl-3-thiosemicarbazide (II) that were dissolved in methanol (Figure 4) and stirred at room temperature. After evaporation of the solvent under reduced pressure and recrystallization from methanol and diethyl ether, the pure derivative 1 was obtained in the form of a yellow solid in 83% yield and melting point in the range 203°C–205°C.
Figure 4: Schematic route for synthesis of Thiosemicarbazone 1.
Photophysical characterization of thiosemicarbazone 1
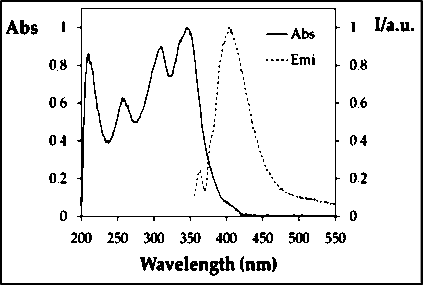
ACN solutions were used to test the photophysical characteristics of Thiosemicarbazone derivative 1. A strong absorption band (log #=4.4) at 346 nm and an emission band (log #=403 nm) were both present in the molecule. 0.05 was discovered to be the relative fluorescence quantum yield when DPA was used as the reference (Figure 5). Other analysis results are also reported in Table 1.
Figure 5: UV-Visible spectra & Fluorescence Spectra of prepared Thiosemicarbazone 1.
Table 1: Analysis results of prepared compound 1.
| Parameter | Result |
|---|---|
| Chemical Name | (Z)-2-((8-hydroxyquinolin-2-yl)methylene)-N-phenyl hydrazine carbo thiomide |
| Chemical Formula | C17H14N4OS |
| Exact Mass | 322.09 |
| Molecular Weight | 322.38 |
| m/z | 322.09 (100.0%), 323.09 (20.7%), 324.08 (4.5%), 324.10 (1.6%) |
| Elemental Analysis | C, 63.33; H, 4.38; N, 17.38; O, 4.96; S, 9.95 |
Preliminary studies of the sensing capacity of the thiosemicarbazone 1
Following the addition of 50 equivalents of the research ions, the Thiosemicarbazone 1 solution in ACN was examined for alterations in colour and fluorescence intensity using “naked eye” visualisation and in a UV-vis chamber using ultraviolet light with a wavelength of 365 nm, respectively.
Chemosensing of anions in ACN
Under daylight, it was possible to see colour changes in compound 1 (from colourless to light yellow) with the addition of BzO- and CN- anions (Figure 6). Compound 1 displayed fluorescence quenching in the presence of BzOand CN- ions as well as variations in_flu for the AcO- ion in terms of fluorimetric behaviour (Figure 7).
Figure 6: Thiosemicarbazone 1 solutions in ACN with several anionic salts under daylight.
Figure 7: Thiosemicarbazone 1 solutions in ACN with several anionic salts under UV light (365 nm).
Chemosensing of cations in ACN
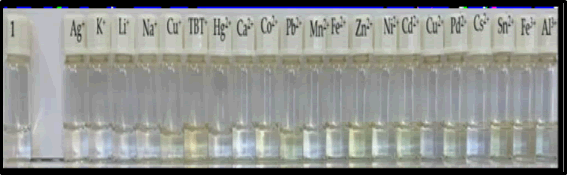
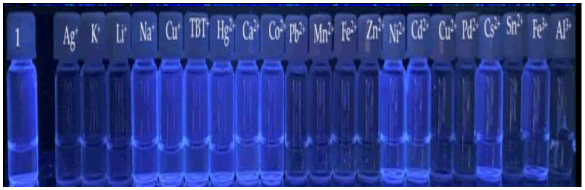
Solutions of compound 1 in ACN experienced color changes (from colorless to light yellow) for Cu+; TBT+, Co2+, Pb2+, Zn2+, Cd2+, Pd2+ and Sn2+ ions, under natural light (Figure 8). Under ultraviolet radiation, there was a quenching of fluorescence in the presence of Ag+, K+, Li+, Pb2+, Mn2+, Fe2+, Zn2+, Cu2+, Pd2+, Sn2+ e Al3+ ions (Figure 9).
Figure 8: Thiosemicarbazone 1 solutions in ACN with several cationic salts under daylight.
Figure 9: Thiosemicarbazone 1 solutions in ACN with several cationic salts under UV light (365 nm).
Antimicrobial activity
The antibacterial activity of Thiosemicarbazone Derivative 1 is shown in Table 2. Thiosemicarbazone Derivative 1 was found to be moderately active against three strains of bacteria. The antibacterial activity of BMM may be attributed to the presence of toxophorically important imine group (-C=N) where the mode of action of such compounds may involve the formation of hydrogen bond through azomethine group with the active centre of the cell constituents, thereby resulting in the interference with normal cell processes. This is the first report of benzylmethyl-4-methyl- 3-thiosemicarbazone on bacteria. Given the promiscuity of Thiosemicarbazones, Thiosemicarbazone Derivative 1 will be screened on other pathogens such as malaria and onchocerciasis. Based upon the achieved results, the Thiosemicarbazone Derivative 1 can be used as antibacterial drug in different medical fields. The potential application of quinoline-functionalized Thiosemicarbazone derivatives as antibacterial drugs is promising. These compounds have shown potent antibacterial activity against various bacterial strains, including multidrug-resistant strains such as methicillin- resistant Staphylococcus aureus (MRSA).
Table 2: Diameter of inhibition zone of bacterial strains by Thiosemicarbazone Derivative 1 at disc potency of 100 μg/mL.
| Compound | Conc (μg/mL) | EC | KP | BC | SE |
|---|---|---|---|---|---|
| Thiosemicarbazone Derivative 1 | 100 | 16 | 14 | NA | 13 |
| Ciprofloxacin (standard) | 100 | 26 | 23 | 26 | 28 |
| NA=Not Active; EC=Escherichi coli; KP=Klebsiella pneumonia; BC=Bacillus cereus; SE=Staphylococcus epidermidis and BMM=benzylmethyl-4-methyl-3-thiosemicarbazone. | |||||
Quinoline-functionalized Thiosemicarbazone derivatives have the potential to be developed as novel antibacterial agents for the treatment of bacterial infections [12]. Due to their broad-spectrum antibacterial activity, they may be used to treat a wide range of bacterial infections, including skin and soft tissue infections, urinary tract infections, respiratory tract infections, and bloodstream infections [13]. Moreover, quinoline-functionalized Thiosemicarbazone derivatives may be used as an alternative to existing antibiotics, especially in cases where bacterial strains have developed resistance to traditional antibiotics. The potential to chelate metal ions may also be exploited in the development of antibacterial agents, as the ability to disrupt metal ion homeostasis is an emerging target for the treatment of bacterial infections [14].
Therefore, quinoline-functionalized Thiosemicarbazone derivatives have the potential to be developed as effective antibacterial drugs for the treatment of bacterial infections [15]. Further research is needed to evaluate their in vivo efficacy and toxicity, and to optimize their pharmacological properties for clinical use.
Conclusion
Through a basic and easy process, a new Thiosemicarbazone derivative 1 functionalized with a quinoline moiety was created in good yield. Spectroscopies for 13C NMR, UV-vis absorption, and fluorescence were used to identify the novel chemical. A preliminary chemosensory study was conducted in acetonitrile solutions in the presence of relevant ions with biological, medicinal, and environmental relevance taking into account the structure of Thiosemicarbazone 1 with various potential binding groups, demonstrating that this receptor has potential use as a colorimetric/ fluorimetric chemosensor. Thiosemicarbazone Derivative 1 was found to be moderately active against three strains of bacteria. Through spectrophotometric and spectrofluorimetric titrations, a more thorough investigation of the interaction of Thiosemicarbazone 1 with the ions that produced the most intriguing findings will be carried out.
Acknowledgement
None.
Conflict of Interest
There are no conflicts of interest among the authors of this study.
Funding
No funding was provided for this study.
Associated Data Availability
There is no associated data with this manuscript.
References
- A.R. Pasha, M. Khalid, Z. Shafiq, M.U. Khan, M.M. Naseer, et al. A comprehensive study of structural, non-covalent interactions and electronic insights into N-aryl substituted thiosemicarbazones via SC-XRD and first-principles DFT approach,J Mol Struct,1230(2021): 129852.
- B.R. Jali, J.B. Baruah, Recent progress in schiff bases in detections of fluoride ions,Dyes Pigm,194(2021): 109575.
- G.M. Abu-Taweel, M.M. Ibrahim, S. Khan, H.M. Al-Saidi, M. Alshamrani, et al. Medicinal importance and chemosensing applications of pyridine derivatives: A review,Crit Rev Anal Chem,(2022): 1-18.
- C. Quiroga-Campano, H. Gómez-Machuca, S. Moris, H. Pessoa-Mahana, C. Jullian, et al. Synthesis of calix [4] arenes bearing thiosemicarbazone moieties with naphthalene groups: Highly selective turn off/on fluorescent sensor for Cu (II) recognition,J Mol Struct,1225(2021): 129125.
- Tarai, Y. Li, B. Liu, D. Zhang, J. Li, et al. A review on recognition of tri-/tetra-analyte by using simple organic colorimetric and fluorometric probes, Coord Chem Rev, 445(2021): 214070.
- C.G.L. Nongpiur, C. Soh, D.F. Diengdoh, A.K. Verma, R. Gogoi, et al. An investigation of the antibacterial, antioxidant and cytotoxicity activities of 3-acetyl-coumarin-substituted thiosemicarbazones and their ruthenium, rhodium and iridium metal complexes,SSRN, (2022): 1-29
- Y.A.M.M. Elshaier, A.A. Aly, M. Abdel-Aziz, H.M. Fathy, A.B. Brown, et al. Synthesis and identification of new n, n-disubstituted thiourea, and thiazolidinone scaffolds based on quinolone moiety as urease inhibitor,Molecules, 20(2022): 7126.
- M. Yıldız, M. Bingul, Y. Zorlu, M.F. Saglam, M. Boga, et al. Dimethoxyindoles based Thiosemicarbazones as multi-target agents; synthesis, crystal interactions, biological activity and molecular modeling, Bioorg Chem,120(2022): 105647.
- K. Kaur, V. Jaitak, Thiazole and related heterocyclic systems as anticancer agents: A review on synthetic strategies, mechanisms of action and sar studies,Curr Med Chem, 29(2022):4958-5009.
- R. Munir, M. Zia-ur-Rehman, S. Murtaza, S. Zaib, N. Javid, et al. microwave-assisted synthesis of (piperidin-1-yl) quinolin-3-yl) methylene) hydrazinecarbothioamides as potent inhibitors of cholinesterases: A biochemical and In silico approach, Molecules, 3(2021): 656.
- A.I. Matesanz, J.M. Herrero, A.G. Quiroga, Chemical and biological evaluation of thiosemicarbazone-bearing heterocyclic metal complexes,Curr Top Med Chem, 21(2021): 59-72.
- S. Zehra, I. Cirilli, S. Silvestri, S. Gómez-Ruiz, S. Tabassum, et al. Structure elucidation, in vitro binding studies and ROS-dependent anti-cancer activity of Cu (II) and Zn (II) phthaloylglycinate (phen) complexes against MDA-MB-231 cells, Metallomics,13(2021): mfab064.
- J. Devi, B. Kumar, B. Taxak, Recent advancements of organotin (iv) complexes derived from hydrazone and thiosemicarbazone ligands as potential anticancer agents,Inorg Chem Commun, 139(2022): 109208.
- S. Cao, D. Wang, R. Cheng, W. Shi, Q. Zhang, et al. Modulation of the lipophilicity and molecular size of thiosemicarbazone inhibitors to regulate tyrosinase activity,Spectrochim Acta A Mol Biomol Spectrosc, 281(2022): 121590.
- L.H. Pineda, E.D. Tecuapa-Flores, J.G. Hernández, P. Thangarasu, J.M.V. Ramos, Ruthenium complex of bis (benzimidazole-yl-ethyl) sulfide as chemo-sensor for selective recognition of chloride ion, and its application in real bacterial samples,Inorganica Chim Acta,522(2021): 120354.
Copyright: © 2023 Waseem Shoukat, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.