Research: Journal of Drug and Alcohol Research (2024) Volume 13, Issue 4
Drug Enhancement of Bioavailability of Nizatidine Drug by using Different Coating Materials
Kameswara Rao S1, Bharghava Bhushan Rao P1*, Chekumuki Venkata Naveen Kumar2, Murikipudi Priya Teja2, Venkata Naga Sai Manikanta2, Jonnalagadda Yamini Priya2 and Vinay Kumar T22Department of Pharmacy, Nirmala College of Pharmacy, India
Bharghava Bhushan Rao P, Department of Pharmacy, AM Reddy Memorial College of Pharmacy, India, Email: drbharghaav28441@gmail.com
Received: 01-Apr-2024, Manuscript No. JDAR-24-132213; Editor assigned: 03-Apr-2024, Pre QC No. JDAR-24-132213 (PQ); Reviewed: 17-Apr-2024, QC No. JDAR-24-132213; Revised: 22-Apr-2024, Manuscript No. JDAR-24-132213 (R); Published: 29-Apr-2024, DOI: 10.4303/JDAR/236292
Abstract
Nizatidine is an effective new H2 receptor antagonist. Nizatidine is a specific, long acting H2 receptor antagonist. It is indicated for the treatment of duodenal ulcer, gastric ulcer, GERD and Zollinger-Ellison Syndrome. In this study 40 mg Nizatidine tablets were formulated and coated with 2 different coating materials i.e. HPMC and opadry white, to check the effect of these coating materials on the bioavailability of Nizatidine tablets. In vitro techniques like disintegration test and dissolution test were used to evaluate Nizatidine tablets. Disintegration test was performed on both the formulations. Mean disintegration time for formulation 1 was found to be 5 minutes and mean disintegration time for formulation 2 was 3 minutes. The results of dissolution after 120 minutes for formulation 1 showed release up to 100.01% and the formulation 2 was released up to 100.05%. For in vivo evaluation these 2 formulations were administered to 8 normal human subjects with 1 week washout period. Blood samples were collected and plasma was obtained and analysized by HPLC. Pharmacokinetic parameters of formulation 1 were Cmax 0.97 μg/ ml ±0.47 μg/ml, tmax was 1.68 hours ± 0.37 hours, AUC 3.97 μg.h/ml ± 1.61 μg.h/ml, AUMC 13.35 μg.h2/ml ± 5.97 μg.h2/ml, MRT 2.27 hours ± 0.55 hours, Ke 0.394 ± 0.052, T1/2 1.97 hours ± 0.38 hours, Vd 5.78 L/Kg ± 4.45 L/Kg, Vss 1.92 ± 0.68 L/Kg, Cl 2.08 ml/h/Kg ± 0.08 ml/h/Kg and for formulation 2 these values were 1.64 μg/ml ± 1.02 μg/ml, 1.5 hours ± 0.46 hours, 10.07 μg.h/ml ± 0.21 μg.h/ml, 11.06 μg.h2/ml ± 0.64 μg.h2/ml, 2.38 hours ± 0.99 hours, 0.394 ± 0.07, 1.97 hours ± 0.69 hours, 6.309 L/ Kg ± 2.72 L/Kg, 1.61 L/Kg ± 0.118 L/Kg, 1.76 ml/h/Kg ± 0.037 ml/h/Kg respectively. Statistical analysis was performed and it was found that the formulation 2 which was coated with opadry white was more bioavailable than formulation 1 which was coated with HPMC. It was also concluded that coating materials affect the bioavailability of Nizatidine tablets.
Keywords
Nizatidine; Bioavailability; Pharmacokinetics; HPMC; Opadry white
Introduction
The Histamine Type-2 Receptor Antagonists (H2RAs) have made a significant impact on the prevention and management of gastroesophageal reflux and ulcers. This class includes cimetidine, ranitidine, famotidine, and nizatidine. Cimetidine, the first H2RA available, has largely been replaced by the newer agents in the class due to its adverse effect profile and the potential to cause significant drug interactions. The other H2RAs are considered equivalent [1-3].
Nizatidine is a relatively new H2 receptor antagonist that is 7.5 and 20 times more potent than ranitidine and cimitidine, respectively in inhibiting basal and pentagestrin-stimulated gastric acid secretion in adults [4].
Excepients are added to the formulation to produce certain properties to the drug and dosage form. Some of these properties of the excepients are used to improve the compressibility of the active drug, stabilize the drug against degradation, decrease gastric irritation, control the rate of drug absorption increase drug bioavailability etc. Excepients in a drug product may also affect the dissolution kinetics of the drug. Excepients may be added intentionally to the formulation to enhance the rate and extent of drugs absorption or to delay or slow the rate of drug absorption. Excepients in a formulation may interact directly with the drug to form a water soluble or water-insoluble complex, e.g., if tetracycline is formulated with calcium carbonate, an insoluble complex of calcium tetracycline is formed that has a slow rate of dissolution and poor absorption. Several studies show that changing the excipients in a formulation changes the bioavailability and pharmacokinetics of the active drug [5].
Coating materials particularly shellac will crosslink upon aging and decrease the dissolution rate. Viscosity of film former used in coating affects the disintegration rate of tablets [5]. The aim of the study is the in vitro-in vivo evaluation of the 2 coating materials i.e. HPMC (Non Aqueous formula) and Opadry white (Aqueous formula).
Materials and Methods
Chemicals
Nizatidine (Aurobindo pharma, Hyd), Lactose (Merck, Mumbai), Carboxymethyl cellulose (Merck, Mumbai), Starch (Merck, Mumbai), Magnesium Stearate (Merck, Mumbai), Talc (Merck, Mumbai), Cellulose Acetate Phthalate (Merck, Mumbai), Propylene Glycol (Merck, Mumbai) Methylene Chloride (Merck, Mumbai), Alcohol (Merck, Germany), Hydroxypropyl Methyl Cellulose (Merck, Mumbai), Propylene Glycol, USP (Merck, Mumbai), Ethyl Alcohol, 200 proof (Merck, Mumbai), Acetonitrile (Merck, Mumbai), Disodium Hydrogen Phosphate (Sigma, Germany), Sodium Dodecyl Sulphate (Merck, Mumbai), Opadry White (Colorcon Ltd. England).
Preparation of formulations
A batch of Nizatidine tablets (400 tablets) was prepared by wet granulation method with single punch machine (Karnavathi).
Each tablet for oral administration contained 40 mg Nizatidine.
Nizatidine 16 g
Lactose 20 g
Carboxymethyl Cellulose 6 g
Starch for paste 2.6 g
Magnesium Stearate 1 g
Talc 1 g
Dried starch to make 52 g
Method of preparation
Weigh Nizatidine, lactose and carboxymethyl cellulose individually and pass them through sieve #16 (0.85 mm) and then placed in a tray. Weigh 2.6 g of starch and dissolve in 10 ml distilled water. Boil about 40 ml of water separately. Add the suspension of the starch in the boiling distilled water until paste was formed. Now add the starch paste in the dried ingredients and mix them for 15 minutes in a cube mixer until wet mass was formed. Pass the semisolid mass through the sieve #10 (2 mm). Dry the granules in fluidized bed dryer (Lab India) at 60°C for 10 minutes. Add magnesium stearate and talc in the granules and compress the tablets by single punch machine.
These tablets were divided into 2 groups. Formulation 1 i.e., half of the tablets (200 tablets) were coated with HPMC, which is non aqueous formula, and remaining half of the tablets (200 tablets) i.e., formulation 2 was coated with Opadry white, which is an aqueous formula, by pan coating method.
Coating Formulation #1
Hydroxypropyl Methyl Cellulose 4 g
Propylene Glycol, USP 1.2 g
Ethyl Alcohol 45 g
Methylene Chloride Q.S. 100 ml
The polymer was gradually added to ethyl alcohol while the solvent was continuously agitated for 5 minutes by hand. A portion of methylene chloride (apprıximate 20 ml) was added to this suspension to solubilize the polymer. The polyethylene glycol was then added and the remainder of the methylene chloride was added to obtain the proper volume that’s 100 ml. Nizatidine tablets were coated by the pan coating method. Time of coating was 45 minutes and the quantity of coating material used was 100 ml at a temperature 50°C.
Coating Formulation #2
Opadry White 20 g
Distilled Water Q.S. 100 ml
Mix the opadry in distilled water in proportions until a uniform mixture was formed and this mixture was vortexed for 10 mins. Nizatidine tablets were coated by pan coating method. Time of coating was 45 minutes and the quantity of coating material used was 100 ml at a temperature 65°C.
Assay of tablets
Tablets of each formulation were triturated in a mortar to fine powder form. 100 mg of the powder was then dissolved in 100 ml 0.1 N HCl. The solution in the flask was filtered and 2 ml of this solution pipetted out in 100 ml volumetric flask. Volume was made upto 100 ml with 0.1 N HCl and the contents of Nizatidine were determined using spectrophotometer at a wavelength of 265 nm. The analysis was conducted in sets of 6 and the average was then calculated. This method was validated via performing the assay on different days and found reproducibility in the results. Different in process quality control tests (contents uniformity of tablets, weight variation, thickness, diameter and hardness) were performed and results were noted.
In-vitro disintegration studies: The in-vitro disintegration of both the formulations was determined using USP disintegration apparatus 6 vessel appartus (Lab india) using water as disintegration medium. The disintegration time of 2 formulations were compared.
In-vitro dissolution studies: The in-vitro Nizatidine release was determined using USP 2 dissolution apparatus for both the formulation using 0.1 N HCI as dissolution medium and at temperature 37°C ± 0.5°C and paddle speed was set at 100 rpm. The samples were collected at time intervals of 15 minutes, 30 minutes, 45 minutes, 60 minutes, 90 minutes, and 120 minutes. The study was performed on 6 tablets. Collect the samples after a specified period of time as mentioned above from each container of dissolution apparatus. Dilute the sample with 100 ml of dissolution medium and took the absorbance at 265 nm with UV spectrophotometer (Schimazdu). The method was validated by performing on different times in a day and between the days and reproducible results were found.
In-vivo study protocol: In vivo study was conducted according to the randomized 2 way crossover design. Eight healthy, non-smoking adult male volunteers with ages between 22 years and 24 years old (mean=22.62 years) with heights from 154 cm to 169 cm (mean=159.5 cm), and weighing from 56 kg to 61 kg (mean=59.5 kg) participated in the study. The volunteers were divided into 2 groups i.e., group 1 and group 2 with 4 volunteers in each group. Written informed consent was obtained from each volunteer after explaining the nature and the purpose of the study. All were found healthy after performing their complete blood and urine analysis and were not receiving any medication prior 2 weeks and during the study period.
All the 4 volunteers of group one each was administered one tablet (containing 40 mg of Nizatidine) of formulation 1 in random and all the volunteers of group 2 were administered one tablet of formulation 2 individually. After a washout period of one week, each volunteer of group one was given one tablet (containing 40 mg of Nizatidine) of formulation 2 and each volunteer of group 2 was given one tablet of formulation one. Both the formulations were administered with 240 ml of water after an overnight fasting. After 2 hours of dosing each subject was provided with breakfast consisted of 2 scrambled eggs, 4 pieces of toast and one glass of milk. Blood samples of 5 ml volume were collected in syringes at 0 (before dosing), 0.5 h, 1.0 h, 1.5 h, 2.0 h, 3.0 h, 4.0 h, 5.0 h, 7.0 h, 9.0 h, and 11 h after dosing via an in-dwelling cannula placed in the forearm. The plasma was harvested and frozen at -15°C until assayed.
Analysis of plasma nizatidine concentration
The plasma samples were analysed using an accurate and validated reversed phase High Performance Liquid Chromatography (HPLC) method. A Hypersil ODS reversed phase column (5 μm, 250 mm × 4.6 mm ID) was used for the separation. The detector was operated at 267 nm. The mobile phase comprised of (20 mM) Disodium hydrogen phosphate, (50 mM) Sodium dodecyl sulphate and acetonitrile (70:30 v/v) and pH was adjusted at 3.0 with phosphoric acid (85%). Analysis was run at a flow rate of 1.0 ml/min and quantified with peak height. Limit of quantification of plasma famotidine was 75 ng/ml–1500 ng/ml [6].
Prior to injection, famotidine was extracted from the plasma samples according to the following procedure: Extraction procedure was simply based on liquid-liquid extraction method [7]. In the extraction procedure 0.5 ml of the drug solution was spiked with 0.5 ml of the blank plasma in the 2 ml of the centrifuge tube and mixed well, then centrifuged for 10 min. Separated the organic layer (Acetonitrile) by micropipette, filtered by using of the filtration syringe. And the filtrate was taken in polypropylene tubes. 20 μl was injected in to the HPLC injection port by injection syringe. Standard curve was prepared to encompass the anticipated range of plasma Famotidine concentration found in healthy subjects taking Famotidine. Blank plasma was spiked with Famotidine drug solution to give the concentrations of 93.25 ng/ml, 187.5 ng/ml, 375 ng/ml, 750 ng/ml and 1500 ng/ml. The extraction procedure was same as described earlier. Injections of 20 μl were injected and spectra were taken of each concentration. The peak areas were noted for each concentration. The absolute recovery of Famotidine from the extraction procedure was determined at different plasma concentrations (93.5 ng/ml to 1500 ng/ ml) by comparing the peak heights of the drug obtained from extracted plasma samples with those obtained from direct injections of the pure famotidine standards in water of equivalent amounts. The mean recovery from plasma samples was 80.7% ± 7.3% at 93.5 ng/ml and 78.9% ± 4.8% at 1500 ng/ml. (The mean recovery for famotidine as mentioned in literature from plasma samples was 86.7% ± 7.3% at 25 ng/ml and 79.9% ± 4.8% at 200 ng/ ml [6,8]. Calibration curve were constructed by plotting the measured peak area of famotidine vs. concentration of standard dilutions. The intra-day (with in run) and inter-day (between run) accuracy and precision were determined in quadruplicate on 3 separate days.
Data analysis
Pharmacokinetic analysis was performed by using MS Excel Windows Professional XP. Pharmacokinetic analysis was performed by using non-compartmental model. Maximum concentration of Famotidine in serum (Cmax) and times to these concentrations (Tmax) were determined by visual inspection of plasma concentration time profiles. At each time points (t), (Ct/Cmax) × 100%/individual was calculated, and the maximum, median and minimum values across all subjects were determined. These % ages can provide some guidance regarding sampling times that can be used clinically. The area under the concentration time curve from 0 hour-infinity (AUC 0-∞) was calculated by the linear trapezoidal rule using the AUC from 0 hour to last measure concentration (C last) plus C last/Kel where t last is the time of the last measured concentration and Ke is the terminal elimination rate constant. All other pharmacokinetic parameters including t1/2, AUMC, MRT, VD, VSS, and CL were calculated by software developed on Microsoft Excel.
Statistical analysis
Statistical analysis was performed by using SPSS 7. f2 similarity test was used to compare the in-vitro dissolution profile of both the formulations. Paired t-test was used to check the differences between the pharmacokinetic parameters of 2 formulations.
Results and Discussion
In vitro evaluation
Percentages of active ingredients of both the formulations were noted and have been presented in the Table 1. Both formulations of famotidine tablets were analyzed for assay purposes by UV spectrophotomertric method. The percentage of active ingredients in both the formulations was found to be 102.33% ± 0.4% as the same batch of tablets was used for coating by 2 different coating materials. This is in accordance with B.P. Disintegration time for both the formulations was noted and has been presented. Mean disintegration time for formulation 1 was found to be 5.0 minutes ± 0.289 minutes and mean disintegration time for formulation 2 was 3.0 minutes ± 0.183 minutes and it was found that there is highly significant difference (p=0.001) between the 2 formulations at 95% confidence interval. The difference in the mean disintegration time of 2 formulations was due to difference in the coating materials. As the formulation 1 was coated with non-aqueous formula, so its disintegration time was longer than that of formulation 2 which was coated by opadry white (which is an aqueous formula).
Table 1: Quality control parameters of formulation 1 and 2
| Parameters | Formulation 1 | Formulation 2 |
|---|---|---|
| Assay | 102.33% ± 0.4% | 102.33% ± 0.4% |
| Hardness | 4.0 Kg/cm2 ± 0.6 Kg/cm2 | 3.0 Kg/cm2 ± 0.4 Kg/cm2 |
| Disintegration time | 5.0 minutes ± 0.289 minutes | 3.0 minutes ± 0.183 minutes |
| Tablet diameter | 6.4 mm ± 0.4 mm | 6.4 mm ± 0.4 mm |
| Tablet thickness | 1.5 mm ± 0.2 mm | 1.5 mm ± 0.3 mm |
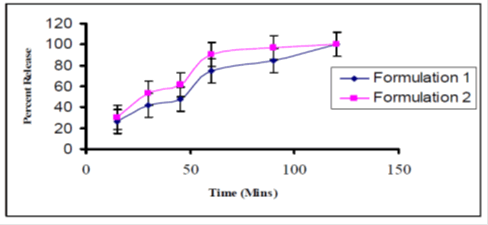
Dissolution profiles of both the formulation have been shown in the Table 2 and represented in the Figure 1. Dissolution tests were performed on both the formulations by calculating their % Co-efficients of Variations (%CV) and found that %CV=39.0423 of formulation 2 was less than %CV=45.1119 of formulation 1 which indicates that in formulation 1 released Famotidine in slower pattern in comparison with the formulation 2 [9]. After 120 minutes formulation 1 was released up to 100.01% and the formulation 2 was released up to 100.05%. On the basis of this comparison it can be concluded that the formulation 2 released the famotidine in a rapid pattern which has been reflected in the differences in Tmax, Cmax and AUC of both the formulations (Table 3). In formulation 2 drugs was released more quickly due to the use of Opadry coating material which is an aqueous coating formula while formulation 1 was released in slower pattern due to the use of HPMC which is a non-aqueous coating formula.
Table 2: Dissolution vs time profile of formulation 1 and 2
| Time (Minutes) | Percent release | |
|---|---|---|
| Formulation 1 | Formulation 2 | |
| 15 | 26.36 | 30.39 |
| 30 | 42.03 | 53.07 |
| 45 | 47.90 | 61.27 |
| 60 | 74.92 | 90.37 |
| 90 | 84.79 | 96.86 |
| 120 | 100.01 | 100.05 |
Table 3: Statistical analysis of pharmacokinetic parameters for formulation 1 and 2
| Parameters | Formulation 1 | Formulation 2 |
|---|---|---|
| Cmax (µg/ml) | 0.97 ± 0.47 | 1.64 ± 1.02* |
| Tmax (hr) | 1.68 ± 0.37 | 1.5 ± 0.46 |
| AUC (µg.h/ml) | 3.97 ± 1.61 | 10.07 ± 0.21* |
| AUMC (µg.h2/ml) | 20.31 ± 5.97 | 16.59 ± 0.64* |
| MRT (hr) | 2.27 ± 0.55 | 2.38 ± 0.99* |
| Ke (Hr-1) | 0.293 ± 0.052 | 0.297 ± 0.07 |
| T1/2 (hr) | 1.97 ± 0.38 | 1.97 ± 0.69 |
| VD (L/Kg) | 5.78 ± 4.45 | 6.309 ± 2.72* |
| Vss (L/Kg) | 1.92 ± 0.68 | 1.61 ± 0.118 |
| Cl (ml/h/Kg) | 2.08 ± 0.08 | 1.76 ± 0.037* |
| *: Significant difference (p<0.05) | ||
Figure 1: Dissolution vs time profile of formulation 1 and 2
FDA’s guiadance on scale up and post approval changes for immediate release oral solid dosage forms (SUPACIR) recommends a metric that can be used to compare dissolution profiles of different formulations for in-vitro tests [10]. This metric f2 is called the similarity factor, here f2 is 48.214 which is less than 50 which means the 2 dissolution profiles are not similar and indicates a pointto- point difference of less than 13%, in the release of Famotidine from formulation 1 and formulation 2 which were compared by using this metric [9]. Dissolution test suggests that both the formulations are not bioequivalent to each other. For a specific formulation and manufacturing process, in vitro tests may be useful to assure lot–to-lot uniformity in bioavailability. However human trials may be necessary to demonstrate that bioavailability remains consistent with a given range of dissolution rate.
In vivo evaluation
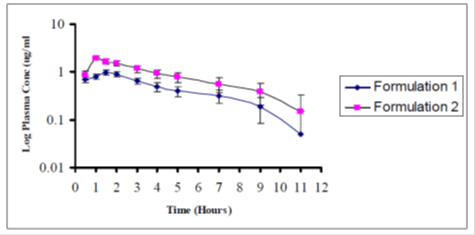
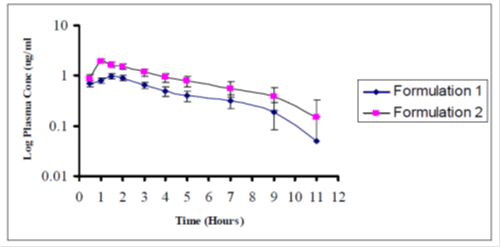
Average plasma concentrations ± SEM versus time for both the formulations have been represented in Figure 2. Average Log plasma concentrations ± SEM versus time for both the formulations have been represented in Figure 3. Both the formulations show fluctuations at certain points. On the average formulation 2 is more bioavailable than formulation 1.
Figure 2: Average plasma concentration ÃÂ?± SEM vs time for formulation 1 and 2 in 8 subjects
Figure 3: Average log plasma concentration ÃÂ?± SEM vs time for formulation 1 and 2 in 8 subjects
All other pharmacokinetic parameters for formulation 1 of all the 8 healthy subjects have been shown.
Several pharmacokinetic parameters observed in our study were comparable to values previously reported in studies of adult subjects. In this study maximum plasma concentrations (Cmax) for formulation 1 were found to be ranging from 0.050 μg/ml-2.61 μg/ml with mean 0.97 μg/ ml ±0.47 μg/ml and for the formulation 2 maximum plasma concentrations (Cmax) were ranging from 0.25 μg/ml-6.14 μg/ml with the mean value 1.64 μg/ml ± 1.02 μg/ml. These values were found to be higher than the values which have already been reported in the literature [11-13]. These differences might be due to change in the population and changes in the body composition of different individual and partly due to the changes in the coating materials used in the formulation development. The mean maximum plasma concentration values for formulation 1 are less than that of formulation 2 which reflects that the formulation 2 will have better pharmacological effects than those of formulation 1. Paired t-test was performed on the average Cmax values for 2 formulations. There was a significant difference between the 2 formulations at 95% confidence interval.
In this study Tmax of the formulation 1 was ranging from 1.0 hour-2.0 hours with mean 1.68 hours ± 0.37 hours and Tmax of formulation 2 was ranging from 1.0 hour-2.0 hours with mean 1.5 hours ± 0.46 hours. These 2 values were found to be less than the values repoted in the pervious studies but these values are consistent with each other [13,14].
Volume of Distribution (VD) for formulation 1 was ranging from 6.69 L/Kg-16.28 L/Kg with mean 5.78 L/Kg ± 4.45 L/Kg and for the formulation 2 was ranging from 6.34 L/ Kg-14.74 L/Kg with mean 6.309 L/Kg ± 2.72 L/Kg. These values are greater than reported in the pervious studies of healthy adults with normal renal function [12-14]. Vss of the formulation 1 was ranging from 2.36-L/Kg with mean 1.92 L/Kg ± 0.68 L/Kg which is a little bit higher than the values of (Vss) for formulation 2 which were ranging from 2.27 L/Kg-2.63 L/Kg with mean 1.61 L/Kg ± 0.118 L/Kg. These values are consistent with different values given in the literature [11]. In this study the plasma half-life of formulation 1 was ranging from 1.72 hours-3.01 hours with mean 1.97 hours ± 0.38 hours and for formulation 2 was ranging from 1.69 hours-3.83 hours with mean 1.97 hours ± 0.69 hours. These values are consistent with the pervious studies conducted on famotidine in different situations [11- 13,15-18].
In this study elimination rate constant i.e. Ke of the formulation 1 was ranging from 0.23-0.4 with mean 0.394 ± 0.052 and for the formulation 2 was ranging from 0.18-0.41 with mean 0.394 ± 0.07. These values are consistent in both these formulations. Clearance (Cl) values for formulation 1 were ranging from 2.66 ml/h/Kg-5.01 ml/h/Kg with mean 2.08 ml/h/Kg ± 0.08 ml/h/Kg and for formulation 2 these values were ranging from 2.59 ml/h/Kg-2.70 ml/h/Kg with mean 1.76 ml/h/Kg ± 0.037 ml/h/Kg. These values were somewhat higher in our study than those values which obtained in pervious studies [14,18].
Conclusion
On the basis of above facts, it can be concluded that aqueous coating materials like Opadry white are more better than non-aqueous coating materials like HPMC for famotidine tablets.
Acknowledgement
None.
Conflict Of Interest
The authors declared that they have no conflict of interest.
References
- N.B. O'Mara, M.C. Nahata, Parenteral histamine 2-receptor antagonists in the pediatric population, J Pharm Tech, 10(1998):53-7.
- B.R. Olin, Drug facts and comparisons, 1994.
- L.P. James, G.L. Kearns, Pharmacokinetics and pharmacodynamics of famotidine in pediatric patients, Clin Pharmacokinet, 31(1996):103-10.
- L.P. James, J.D. Marshall, M.J. Heulitt, Pharmacokinetics and pharmacodynamics of famotidine in children, J Clin Pharmacol, (1996):48-54.
- L. Shargel, B.C. Andrew, S. Wu-Pong, Applied biopharmaceutics and pharmacokinetics, 4th Ed, 1999.
- S. Wanwimolruk, A.R. Zoest, S.Z. Wanwimolruk, C.T. Hung, Sensitive high-performance liquid chromatographic determination of famotidine in plasma application to pharmacokinetic study, J Chromatogr, 572(1991):227-38.
- H. Jalalizadeh, M. Amini, V. Ziaee, A. Safa, H. Farsam, et al. Determination of celecoxib in human plasma by HPLC, J Pharma Biomed Analysis, 35(2004):665-670.
- T.C. Dowling, R.F. Frye, Determination of famotidine in human plasma and urine by high-performance liquid chromatography, J Chromatogr B Biomed Sci Appl, 732(1999):239-43.
- J.W. Moore, H.H. Flanner, Mathematical comparison of dissolution profiles, Pharm Technol, 20(1996):64-74.
- Guidence for Industry, Immidiate release oral solid doses forms, Federal Register, CDER FDA, (1995):61638-61643.
- L.P. James, T. Marotti, C.D. Stowe, H.C. Farrar, B.J. Taylor, et al. Pharmacokinetics and pharmacodynamics of famotidine in infants, J Clin Pharmacol, 38(1998):1089-95.
- L.P. James, J.D. Marshall, M.J. Heulitt, T.G. Wells, L. Letzig, et al. Pharmacokinetics and pharmacodynamics of famotidine in children, J Clin Pharmacol, 36(1996):48-54.
- H. Kroemer, U. Klotz, Pharmacokinetics of famotidine in man, Int J Clin Pharmacol Ther Toxicol, 25(1987):458-63.
- W.A. Maish, M.M. McCubbin, L.G. Letzig, H.C. Farrar, G.L. Kearns, Pharmacokinetics of famotidine in patients with cystic fibrosis, J Clin Pharmacol, 38(1998):1010-6.
- G.K. McEvoy, American hospital formulary service-drug information,1996.
- S. Miyake, M. Yamada, H. Iwamoto, S. Yamashita, Y. Sugio, Effect of a new H2-blocker, famotidine, in reflux esophagitis among severely handicapped children, Clin Ther, 9(1987):548-58.
- American Medical Association, AMA Drug Evaluations Annual, 1994.
- S. Gao, G.L. Liu, S.X. Wang, X.H. Gao, Pharmacokinetics and bioavailability of famotidine in 10 Chinese healthy volunteers, 12(1991):195-8.
Copyright: © 2024 Kameswara Rao S, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.