Case Report: Journal of Drug and Alcohol Research (2025) Volume 14, Issue 1
Bilateral Presentation of Rasmussen’s Encephalitis: Case Report Responding to Drug Therapy and Comprehensive Review
Lourdes de Fatima Ibanez Valdes1 and Humberto Foyaca Sibat2*2Department of Neurology, Nelson Mandela Academic Hospital, Walter Sisulu University, South Africa
Humberto Foyaca Sibat, Department of Neurology, Nelson Mandela Academic Hospital, Walter Sisulu University, South Africa, Email: humbertofoyacasibat@gmail.com
Received: 20-Mar-2025, Manuscript No. JDAR-25-162719; Editor assigned: 25-Mar-2025, Pre QC No. JDAR-25-162719; Reviewed: 08-Apr-2025, QC No. JDAR-25-162719; Revised: 22-Apr-2025, Manuscript No. JDAR-25-162719; Published: 29-Apr-2025, DOI: 10.4303/JDAR/236428
Abstract
Rasmussen’s Encephalitis (RE) stands as a very uncommon neurological condition marked by refractory epilepsy to Antiepileptic Drugs (AED) typically emerging during childhood, marked by a progressive unilateral hemispheric degeneration of the brain leading to a gradual cerebral hemiatrophy which is increasingly recognised as an autoimmune mediated disorder. Despite extensive investigations, the actual aetiology of RE remains elusive, while its histopathological features encompass neuronal degeneration, cortical inflammation and involvement of the supporting cells. We hypothesised about the underlying molecular mechanisms, which have been largely unexplored. We report a patient presenting pathological involvement of the bilateral cerebral hemisphere who has remained seizure-free for over six months under Antiepileptic Drugs (AED) and is also experiencing mild motor improvement. We searched comprehensively the medical literature and we did not identify a similar case.
Keywords
Rasmussen’s encephalitis; Rasmussen; Cell cycle; Inflammatory; Encephalopathy; BDNF; Brain-derived neurotrophic factor; Drug therapy
Introduction
Rasmussen Encephalitis (RE) is an uncommon chronic Neuroinflammatory (NI) encephalopathy. The clinical features encompass hemiplegia, impairments in motor skills and speech, severe uncontrolled recurrent epileptic seizures, dementia and the encephalitis condition marked by brain NI leading to progressive atrophy of one cerebral hemisphere [1].
The aetiology of RE is still unknown; histopathological features include neuronal loss, gliosis localised to one cerebral hemisphere and cortical inflammation, whereas the participation of T lymphocytes has been described [2,3]. Early diagnosis of RE is mandatory to begin a proper therapy aimed at arresting disease progression and ameliorating case outcomes [4]. Therefore, developing novel biomarkers and non-invasive therapies is also mandatory to improve our understanding of the disease’s molecular mechanisms [1].
Some patients presenting RE also suffer from Epilepsia Partialis Continua (EPC), which may emerge on any given day during RE, with rates of 37% to 92% within the first 3 to 5 years of the beginning of RE. It is reported by the International League Against Epilepsy (ILAE) (https:// www.epilepsydiagnosis.org/, accessed on 02 February 2025) as “recurrent focal motor seizures (typically affecting face and hand, although other parts of the body parts may be involved) that happen every few seconds minutes for extended periods (days or years)”.
Here, we report a case with RE, who was treated with antiepileptic drugs and remained seizure-free for several months, with progressive motor improvement. Some authors made a molecular analysis of resected brain tissue and reported a down-regulation of cell-cycle-related genes, possibly due to increased BDNF protein levels, which will be commented on later.
In contrast to many types of cells, NC are thought to lose their ability to multiply once matured, remaining quiescent within the CNS. However, reactivation of the cell cycle in adult neurons is an initial indicator of neurodegeneration and CNS injury [5]. Several studies suggest that neuronal death in conditions such as seizures, ischemia and CNS diseases involve PCD, including apoptosis [6]. Brain Derived Neurotrophic Factor (DNF) is a protein that belongs to the neurotrophins family and plays a crucial role in the maintenance, survival and growth of neurons in the brain [7]. It has been documented that BDNF protein levels are increased in RE brain tissue. In the context of RE, an initial injury could lead to an increase in BDNF as a neuroprotective response. When excessive, BDNF inhibits cell cycle regulators, including apoptosis-related genes, thus causing the survival of damaged neurons [8].
Many signalling pathways are integral to the BDNF protein. Neurotrophins are highly involved in neurogenesis, axonal growth, synaptic plasticity, network transmission, neuroplastic processes and brain maturation. Those damaged NC in RE then stimulate the production of more BDNF, creating a feedback loop. This loop amplifies neurodegeneration, ultimately worsening the RE.
In their first description, Rasmussen and colleagues considered that a viral infection caused this pathological condition. Later, the pathological process was linked to haematological autoantibodies and currently, it was shown that the cause of RE was a cytotoxic T-cell reaction to neurons [9]. Histopathological, RE is identified by cerebral hemispheric-specific gliosis, cortical inflammation and NC degeneration and increasing multilocular inflammation is seen in the affected cerebral hemisphere [9]. Now, RE-related seizures are best managed by surgical approaches such as complete hemispheric disconnection (hemispherectomy). Notwithstanding, thalidomide as a first-line pharmacological therapy has been reported with good results in anecdotic cases [9].
Quantitative histopathology investigations documented that this development is associated with reactive astrocytes and reduced T cells [10]. These findings lead to an initial, active inflammation and eventually “burns out” associated with Epilepsia Partialis Continua (EPC), an intractable partialonset seizure. Destruction of the cerebral hemisphere is the clinical hallmark of RE. Most clinicians who treat RE cases consider immune drug therapy before hemispherectomy. However, the scanty information on the long-term history of untreated RE patients explains why there are so many uncontrolled clinical trials in RE [11].
Search strategies
We comprehensively searched the electronic databases Scopus, Embase, Google Scholar, PubMed, MEDLINE, Research Gate, and the English/Portuguese/Spanish language medical literature.
Inclusion criteria
Peer-reviewed journals produced in English, Portuguese and Spanish articles published in the last twenty years, the full text of the publication, type of publication: Systematic review, review articles, meta-analysis or empirical studies published in peer-reviewed scientific journals, compliance with the combinations of keywords: Treatment advances, targeted therapy, clinical features, pathobiology and the neurological manifestation.
Exclusion criteria
Articles published earlier than twenty years, lack of full text of the publication, language of the publication different than English/Portuguese/Spanish, type of publication other than systematic review, meta-analysis, review articles or empirical studies published in peer-reviewed scientific journals, specific treatment protocol, co-morbidities along with the diseases affecting presentation or than that of the illness in review.
Key terms used for the search are “Rasmussen” (all fields) OR “Rasmussen s”(all fields) AND “encephalitis” (all fields) OR “encephalitis” (MeSH terms) OR “encephalitis” (all fields) OR “Response to drugs therapy”.
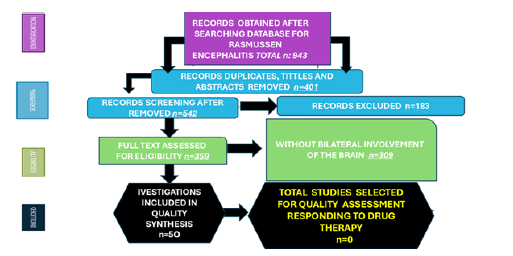
We identified 943 publications related to RE, after removal 401 articles due to duplication and other reasons, we review the titles and abstracts of 502 publications. 183 records were excluded because their inclusion/exclusion criteria. A total of 359 were finally selected without taken into consideration the affected side of the brain, finally only 50 articles included bilateral damage of the brain, but none reported motor improvements and good response to the antiepileptic drugs. The PRISMA method used in the research methodology is depicted in Figure 1 and Table 1.
Figure 1: PRISMA. Flow diagram with included publications.
| From | DR. JSS | ||||
| To | DR G | ||||
| Date | 2024/07/27 | ||||
| RE | LH | Age | 25 Y/O | ||
| Hosp. no. | NMA – 185xxxxxx | Gender | Male | Wt. | 72 kg |
| Diagnosis | Rasmussen encephalitis | ||||
| ICD 10 | |||||
Table 1: Details of the patient
Case Presentation
A 25-year-old male was referred to Nelson Mandela Academic Hospital (NMAH) Neurology from Mthatha Regional Hospital Mental Health Care Unit (MRHMHCU).
Background history
Uncontrolled seizures since 2016 and presenting crisis lasting more than 24 hours with a focal origin like epilepsy partialis continua.
Multiple admissions for epilepsy at a district hospital; the latest admission was in 2020 for status epilepticus.
Previous treatment:
• Na Valproate (EPILIM) CR 500 mg BD
• Carbamazepine 200 mg BD
Both drugs were at therapeutic levels. He was then referred to the mental health care unit from the district hospital due to progressively worsening forgetfulness (phone passwords, bank PINs, etc.). He eventually dropped out of school and developed suicidal ideation and depressive symptoms. MHCU referred him to neurology, where he reported having 3 seizures per week despite being compliant with treatment.
On examination
MSE: Mild depression for psychotherapy MMSE=29/30 Cranial nerves/Motor/Sensory/Coordination/GAIT–NAD
Investigations
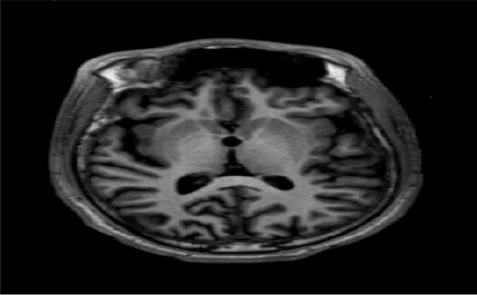
CTB: Asymmetrical bilateral cerebral atrophy on the right with cross atrophy to the left cerebellum. Findings in keeping with asymmetrical bilateral cerebral atrophy (Figure 2). Differentials include Rasmussen’s encephalitis and postictal cerebral hemiatrophy.
Figure 2: Differentials include Rasmussen’s encephalitis and postictal cerebral hemiatrophy
http://umthata.frisdns.com:8080/jivexmobile?code=YKNOG- 326YA-48EL2
password - 1999-06-07
MRI: Unilateral cortical atrophy of the right cerebral hemisphere with associated ex vacuo dilatation on T1.
Asymmetrical atrophy of the right cerebral and left cerebellar hemispheres as in the CTB.
Nil features to suggest Sturge Weber/hemi convulsionhemiplegia epilepsy syndrome.
DDX: Rasmussen’s Encephalitis
http://umthata.frisdns.com:8080/jivexmobile?code=3U- 5WV-SKE7Q-VRXQL
He was counselled extensively, and his dosages were adjusted as follows:
• Na Valproate (EPILIM) CR 1 g BD po
• Acetazolamide 500 mg TDS po
• Clonazepam (RIVOTRIL) 0, 5 mg NOCTE po
• Folic acid 5 mg DAILY po
Upon review, 4 months later, his seizures were much more controlled and improved from 3 x seizures per week to 2-3 x per month, and the muscle power improved on the affected side from 2/5 to 4/5.
Comments and final remarks
As we cited previously, RE is characterised by unilateral hemispheric brain shrinkage, cognitive deterioration, drugresistant focal epilepsy and progressive hemiplegia. The condition is rare and mainly affects kids and young adults. Leach et al. reported that only 24 cases per 10 million children and teenagers may be seen countrywide each year [12]. However, other authors calculated an incidence of 17 per 10 million children below 16 years old [13].
On the other hand, no investigations have confirmed any gender, geography or ethnic predominance and the onset age range spans from childhood through adulthood, with an average age of 6 years. Around eight individuals may have a prodromal phase of moderate hemiparesis with occasional seizures years before the acute event. As this pathological condition worsens, several partial seizure semiologists suggest newly infected sites of inflammation in the hemisphere. When the language-dominant hemisphere is affected, patients will experience hemianopia, dysphasia, hemiparesis and cognitive deterioration within a year of the onset of epilepsy [14].
RE may present a variety of clinical features, and around 10% of reported cases begin in youth or adulthood [13]. The clinical features are often delayed and the deficits are more severe in children than adults. Unilateral involuntary movement can be hemi dystonia and hemichorea. The existence of bilateral illness is debatable. However, it is most likely very uncommon [15].
Around two of the roughly half a thousand cases of RE that have been described have histological proof of bilateral disease. Seizures are not a significant side effect of RE [16]. Most scientific community agrees that several CNS illnesses are linked to dangerous circulating Ab to NC surface proteins [17]. Moreover, in some cases of RE, GluR3 Ab and Munc-18 (intracellular protein necessary for synaptic vesicle discharge) have been reported [18].
Results and Dicsussion
T-cell cytotoxicity
We hypothesized that the role played by T lymphocytes in the pathogenesis of RE is vital because Around 10% of inflammatory T lymphocytes are CD8-positive, which contain granzyme B. Granzyme B cells contain cytotoxic granules directed toward the target membrane of the NC and astrocytes as has been proposed by other authors [19,20]. We also hypothesized that these cytotoxic T lymphocytes can identify foreign antigens from pathogeninfected astrocytes and NC rather than autoantigens, and for the other hand, some infections attacking specific areas of the brain may explain the occurrence of RE based on the finding of coexistence of viruses such Cytomegalovirus, Epstein-Barr virus, enterovirus.
Moreover, herpes simplex in the brain tissues of cases presenting RE has been documented by other investigators [19]. Regarding the inflammatory gene activity, some researchers reported that the RE cohort had higher activity levels of a subset of functionally essential elements, such as interferon- CXCL9, CXCL10, CCL5, CCL22, CCL23, and Fas ligand, than did the cortical dysplasia group of patients. Nonetheless, several genes include those that encode specific chemokines connected to the expression of inducer T lymphocytes and T helper and memory and effector T cells, which help draw these T cells to the site of inoculation [21].
The role of microglia-induced degeneration
We documented several times the role of Microglia (Mg) polarization on the pathogenesis of some neuropathological processes [22,23]. We also consider that Mg activation plays a crucial role in the pathogenesis of RE in both directions via M1 with the production of proinflammatory elements and M2 with anti-inflammatory components. In the case of RE, Mg can produce ES by releasing proinflammatory cytokines, interleukin and increasing network excitability, which has been supported by other authors [24]. The exact way Mg cells are involved in RE’s pathogenesis is still being determined. Along with Mg, as expressed in RE, the pattern of astroglia expression strongly resembles the advance of cortical damage [25].
Astrocytes have a remarkable role in the immune response in RE. Nevertheless, as the pathological process advances, the as are destroyed, most likely due to a cytotoxic T-cell response. The CSF protein level also increases while the disease progresses and the inward-rectifying potassium channels in as are downregulated after protein absorption, which decreases the buffering of extracellular K and NMDA receptor-mediated NC excitability. As a result, protein leakage through as regulation leads to seizures starting during RE [26].
RE immune agents
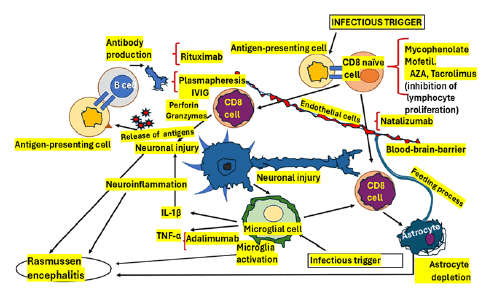
Based on that pathophysiology, many immune-stimulating drugs have been recommended to treat RE cases. However, after searching the medical literature, we found only a few well-designed clinical studies on the immunological approach to RE and most of them administered Intravenous Immunoglobulin (IVIG) and corticosteroids (methylprednisolone taken at night) mainly in the acute stage of the disease [27]. This drug therapy can affect the stability of other genes’ mRNA, protein synthesis and the transcription of specific proinflammatory genes such as IL- 1, IL-2, IL-6, TNF alpha and adhesion molecules [28,29]. The pathogenetic model of RE is graphically shown in Figure 3.
Figure 3: Pathogenetic model of Rasmussen encephalitis
One of the drugs working on the pathogenesis of RE included in Figure 3 is Tacrolimus, which was linked to a similar clinical response to IVIG therapy but with a higher risk of adverse reactions. We also include here the purine synthesis inhibitor Azathioprine (AZA), which has shown the capacity to improve seizure frequency without protective effects on the progression of cognitive decline. In Figure 3, we also included Mycophenolate Mofetil (MMF), which has been successfully used in many juvenile cases of autoimmune encephalitis, leads to fewer side effects than AZA and can block the elaboration of purine in lymphocytes, leading to Programmed Cell Death (PCD), in expressed T lymphocytes, diminishing recruitment of lymphocyte [30].
Despite thalidomide has been prescribed anecdotally and some improvement in the epileptic seizure frequency reported [31], we could not find enough confident information to allow us to recommend its use. Currently, seems that functional hemispherectomy remains the only confident treatment option to control refractory epilepsy despite advances in our knowledge of the immunological causes of RE [32]. Quite often, drug therapy fails many times to reduce seizure frequency or delay the progress of this pathological process. Some benefits from immunetargeting drugs and more may be developed. Drug therapy for similar grey matter disorders might help treat RE; however, because of the rarity of RE, global collaboration studies are needed for the prospective collection of archival cases and enrolment in clinical trials. Forecasting how drug therapy will progress until the aetiologies of RE are discovered is almost impossible to establish. Even though an essential number of immunotherapy programs have been launched over the last ten years, it has been remarkably insufficient to clarify the pathogenesis of RE. However, we believe that our hypotheses on the pathogenic mechanism of RE can be validated until further investigation confirm or deny it.
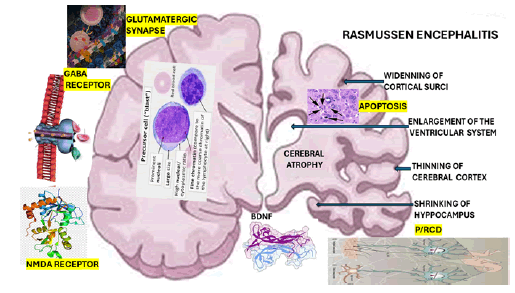
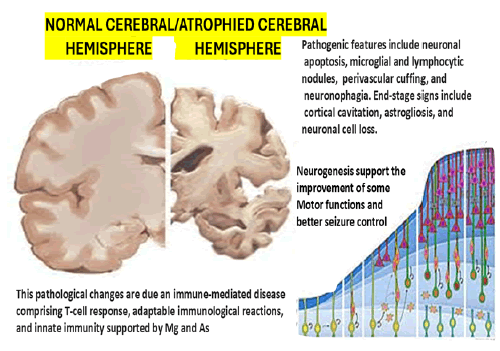
As we mentioned before, Brain-Derived Neurotrophic Factor (BDNF) develops an essential influence on the pathophysiology of network transmission by bolstering excitatory neurotransmitters on glutamatergic synapses and tendentially weakening inhibitory synapses, also known as GABAergic synapses [33]. It has been documented that the excitatory glutamatergic synapse is a significant source of BDNF in the brain. The BDNF effects on synaptic transmission in the brain are graphically represented in Figure 4. In 2020, Tomas et al. demonstrated BDNF gene activity in GABAergic interneurons of the hippocampus and neocortex, which is severely damaged in RE [34] and where its highest concentration is found, critically involved in learning and memory processing [33].
Figure 4: Graphical representation of Rasmussen encephalitis
On the pre-synaptic region, BDNF-trkB signalling enhances glutamate release, thus enhancing the frequency of excitatory post-synaptic currents in hippocampal neurons; in contrast, in the post-synaptic area, BDNF-trkB signalling increases the function of ionotropic glutamate receptors, which promotes long-term modifications of synaptic activity [34]. On the other hand, BDNF modulates the signalling cascades downstream of neuronal excitation, phosphorylation, late effects in synaptic plasticity (gene transcription), trafficking, local protein synthesis, the activity of N-methyl-D-aspartate (NMDA) receptors related to enhanced synaptic expression and reduce inhibitory GABAergic synaptic transmission (hippocampus), partly by direct downregulation of Cl-transport or through GABAA receptor modulation [33,35].
On the other hand, we hypothesized that the clinical improvement seen in some cases after drug therapy might be related to the BDNF effects on neurogenesis, mainly in the sub granular zone of the dentate gyrus in the hippocampus, the subventricular zone and the periventricular area of the spinal cord with the new generation of NC, As, and/or oligodendrocytes from the comprises Neural Precursor Stem Cells (NPSCs) [22,23,33]. These NPSCs may proliferate and differentiate into neuroblasts at the before-cited regions and then migrate to the olfactory bulb before becoming interneurons and after maturing, move into excitatory granule neurons, which will move to the granular layer and integrated into hippocampal network circuits with essential implications for memory and cognitive function [36]. We studied the signalling mechanisms involving the activation of trkB, MAPK and PI3-kinase pathways, the inhibition of caspase-3-induced apoptosis and downstream variations of transcriptional factors which can implicate the neurogenic effects of BDNF under different circumstances [37-40].
We hypothesised that BDNF plays a crucial role in PCD/ RCD in cases presenting RE, which explains the progressive brain atrophy seen in those patients, as represented in Figure 4 [41]. The histological features of RE are summarised in Table 2 to reduce the length of this manuscript. To reduce the length of this article, we summarised in Table 3 the most remarkable histopathological description of each RE group’s histological components proposed by Robitaille et al. in 1991, documented by Capara et al. [42] and modified by us. Our final comment on the outcome of our case is related to the role of neurogenesis, which can explain the gradual recovery of motor function and decrease in the frequency of ES despite the severity of the brain damage represented in Figure 5. We hypothesized the neurogenesis promoted by BDNF is the main reason for the improvement.
| Drug | Mechanism of action |
Clinical implications |
|---|---|---|
| Intravenous immunoglobulin | Immune control through several antibody-free routes (cytokine modulation, complement downregulation) |
Usually in conjunction with steroids medication. Varying effectiveness, ranging from short-term, partial responses to long-term responses |
| Corticosteroids (mainly high-dose methylprednisolone) | The broad-spectrum post-transcriptional and transcriptional anti-inflammatory action. Modulation of the expression of adhesion molecules such as cytokines |
Often switching between IVIG and other immunosuppressive drugs. Effective during the short term |
| Tacrolimus | Calcineurin blocker reduces IL-2 synthesis, essential for T-cell activation and proliferation |
It is effective in delaying the onset of illness but ineffective in managing seizures. More serious adverse events than with IVIG |
| Azathioprine | Creating a purine analogy as a purine synthesis inhibitor (6-mercaptopurine) reduces lymphocyte clonal growth |
It is effective in reducing seizures but ineffective in delaying or stopping disease development |
| Thalidomide | Inhibition of innate and adaptive immune responses through a variety of ways. Indirect inhibition of NF- |
Not a viable first-line choice |
| Rituximab | Chimeric anti-CD20 monoclonal antibody. Targets B cells, inhibiting their proliferation and reducing antibody production |
Partial effectiveness in halting disease development and symptom progression. Excellent safety profile |
| Adalimumab | A monoclonal anti-TNF antibody with humanization |
Effective against seizures; ineffective against cognitive deterioration |
| Natalizumab | Antibody against the alpha 4 integrin that has been humanized reduces T cells' capacity to traverse the BBB |
Promising outcomes in terms of safety and effectiveness |
| Mycophenolate mofetil | Inhibitor of inosine monophosphate dehydrogenase, which inhibits the production of purines, decreases lymphocyte recruitment and proliferation, and causes the death of activated T lymphocytes |
Promising outcomes in terms of safety and effectiveness |
Table 2: Pharmaceutical drugs acting on the immune system used in RE
|
Group 1: Inflammation with plenty of microglial nodules, with or without neuronophagia, glial scarring and perivascular round cells |
|
Group 2: Many cuffs of perivascular round cells, microglial nodules, and at least one gyrus segment of complete necrosis |
|
Group 3: Gliosis and neuronal loss with few microglial nodules and abundant perivascular round cells |
|
Group 4: Mild perivascular inflammation, scanty microglial nodules, poor neuronal loss, and various degrees of glial scarring and gliosis |
Table 3: Description of each RE group's histological components.
Figure 5: Severity of brain damage.
Our patient complained of mild clinical manifestation of anxiety, which did not require psychiatry management or even a low dosage of anxiolytic drugs and it improved along with better control of the seizure frequency and progressive improvement of the motor activities. Recently, Maravi and collaborators have established that psychiatric manifestations are often overlooked in cases with RE and present a prominent psychiatric symptom, including psychotic features and behavioural disturbances associated with developmental delays and longstanding seizures [43].
Conclusion
Because RE with bilateral cerebral damage is less common than the unilateral one, like our case, it is more difficult to find abundant publications regarding this condition. Therefore, it will be more problematic to identify many cases presenting this problem to bring more knowledge for a better understanding of the pathogenesis of this disease and to support or deny our hypotheses.
Acknowledgements
We thank Dr Sibi Joseph for elaborating on the patient discharge summary.
Author Contributions
Both authors made a similar contribution to this study and read and agreed with its contents.
Institutional Review Board Statement
The institution’s ethical committee conducted this study at Nelson Mandela Academic Hospital in Mthatha, South Africa.
Informed Consent Statement
The participant signed a free and informed consent.
Data Availability Statement
All data from this article are available from the corresponding author under reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
References
- T. Rasmussen, J. Olszewski, D. Lloydâ?Smith, Focal seizures due to chronic localised encephalitis, Neurology, 8(1958):435-445.
[Crossref] [Google Scholar] [PubMed]
- J.I. Gonçalves, V.R. de Castro, W.A. Martins, F.A. Xavier, J.C. Da Costa, et al. Case report: Molecular analyses of cell-cycle-related genes in cortical brain tissue of a patient with Rasmussen encephalitis, Int J Mol Sci, 25(2024):8487.
[Crossref] [Google Scholar] [PubMed]
- N. Schwab, C.G. Bien, A. Waschbisch, A. Becker, G.H. Vince, et al. CD8+ T-cell clones dominate brain infiltrates in Rasmussen encephalitis and persist in the periphery, Brain, 132(2009):1236-1246.
[Crossref] [Google Scholar] [PubMed]
- C.G. Bien, J. Schramm, Treatment of Rasmussen encephalitis half a century after its initial description: Promising prospects and a dilemma, Epilepsy Res, 86(2009):101-112.
[Crossref] [Google Scholar] [PubMed]
- J.M. Frade, M.C. Ovejero-Benito, Neuronal cell cycle: The neuron itself and its circumstances, Cell Cycle, 14(2015):712–720.
[Crossref] [Google Scholar] [PubMed]
- H. Chi, H.Y. Chang, T.K. Sang, Neuronal cell death mechanisms in major neurodegenerative diseases, Int J Mol Sci, 19(2018):3082.
[Crossref] [Google Scholar] [PubMed]
- C.S. Wang, E.T. Kavalali, L.M. Monteggia, BDNF signaling in context: From synaptic regulation to psychiatric disorders, Cell, 185(2022):62-76.
[Crossref] [Google Scholar] [PubMed]
- N. Boutahar, E. Reynaud, F. Lassabliere, J. Borg, Brain-derived neurotrophic factor inhibits cell cycle reentry but not endoplasmic reticulum stress in cultured neurons following oxidative or excitotoxic stress, J Neurosci Res, 88(2010):2263–2271.
[Crossref] [Google Scholar] [PubMed]
- A. Kumar, H. Krishnani, A. Pande, S. Jaiswal, R.J. Meshram, Rasmussen’s encephalitis: A literary review, Cureus, 15(2023):e47698.
[Crossref] [Google Scholar] [PubMed]
- C.G. Bien, J. Bauer, T.L. Deckwerth, H. Wiendl, M. Deckert, et al. Destruction of neurons by cytotoxic T cells: A new pathogenic mechanism in Rasmussen's encephalitis. Ann Neurol, 51(2002):311-318.
[Crossref] [Google Scholar] [PubMed]
- H. Oguni, F. Andermann, T.B. Rasmussen, The syndrome of chronic encephalitis and epilepsy. A study based on the MNI series of 48 cases, Adv Neurol, 57(1992):419-433.
- J.P. Leach, D.W. Chadwick, J.B. Miles, I.K. Hart, Improvement in adult-onset Rasmussen's encephalitis with long-term immunomodulatory therapy, Neurology, 52(1999):738-742.
[Crossref] [Google Scholar] [PubMed]
- K. Lamb, W.J. Scott, A. Mensah, Prevalence and clinical outcome of Rasmussen encephalitis in children from the United Kingdom, Dev Med Child Neurol, 55(2013):14.
- C.G. Bien, U. Gleissner, R. Sassen, G. Widman, H. Urbach, et al. An open study of tacrolimus therapy in Rasmussen encephalitis, Neurology, 62(2004):2106-2109.
[Crossref] [Google Scholar] [PubMed]
- M.G. Bhatjiwale, C. Polkey, T.C. Cox, A. Dean, N. Deasy, Rasmussen's encephalitis: Neuroimaging findings in 21 patients with a closer look at the basal ganglia, Pediatr Neurosurg, 29(1998):142-148.
[Crossref] [Google Scholar] [PubMed]
- I. Korn-Lubetzki, C.G. Bien, J. Bauer, Rasmussen encephalitis with active inflammation and delayed seizures onset, Neurology, 62(2004):984-986.
[Crossref] [Google Scholar] [PubMed]
- A. Vincent, C.G. Bien, S.R. Irani, P. Waters, Autoantibodies associated with diseases of the CNS: New developments and future challenges, Lancet Neurol, 10(2011):759-772.
[Crossref] [Google Scholar] [PubMed]
- T. Granata, L. Fusco, G. Gobbi, Experience with immunomodulatory treatments in Rasmussen's encephalitis, Neurology, 61(2003):1807-1810.
[Crossref] [Google Scholar] [PubMed]
- C.G. Bien, H. Tiemeier, R. Sassen, Rasmussen encephalitis: Incidence and course under randomised therapy with tacrolimus or intravenous immunoglobulins, Epilepsia, 54(2013):543-550.
[Crossref] [Google Scholar] [PubMed]
- F. Longaretti, C. Dunkley, S. Varadkar, F. Vargha-Khadem, S.G. Boyd, et al. Evolution of the EEG in children with Rasmussen's syndrome, Epilepsia, 53(2012):1539-1545.
[Crossref] [Google Scholar] [PubMed]
- Y.M. Hart, F. Andermann, D.R. Fish, Chronic encephalitis and epilepsy in adults and adolescents: A variant of Rasmussen's syndrome? Neurology, 48(1997):418-424.
[Crossref] [Google Scholar] [PubMed]
- L.F.I. Valdes, H.F. Sibat, Novel hypotheses on the role of microglia and PANoptosis in neurocysticercosis, Clin Schizophr Relat Psychoses, 17(2023).
[Crossref]
- L.F.I. Valdes, H.F. Sibat, New hypotheses on the role of microglia in ischemic reperfusion injury secondary to neurocysticercosis, Clin Schizophr Relat Psychoses, 17(2023).
[Crossref]
- C.G. Bien, C.E. Elger, Y. Leitner, Slowly progressive hemiparesis in childhood due to Rasmussen encephalitis without or with delayed-onset seizures, Eur J Neurol, 14(2007):387-390.
[Crossref] [Google Scholar] [PubMed]
- J. Bauer, C.E. Elger, V.H. Hans, J. Schramm, H. Urbach, et al. Astrocytes are a specific immunological target in Rasmussen's encephalitis, Ann Neurol, 62(2007):67-80.
[Crossref] [Google Scholar] [PubMed]
- G.C. Owens, M.N. Huynh, J.W. Chang, Differential expression of interferon-γ and chemokine genes distinguishes Rasmussen encephalitis from cortical dysplasia and provides evidence for an early Th1 immune response, J Neuroinflammation, 10(2013):56.
[Crossref] [Google Scholar] [PubMed]
- P.I. Andrews, J.O. McNamara, D.V. Lewis, Clinical and electroencephalographic correlates in Rasmussen's encephalitis, Epilepsia, 38(1997):189-194.
[Crossref] [Google Scholar] [PubMed]
- J. Vandewalle, A. Luypaert, K. De Bosscher, C. Libert, Therapeutic mechanisms of glucocorticoids, Trends Endocrinol Metab, 29(2018):42-54.
[Crossref] [Google Scholar] [PubMed]
- G. Ferrara, M.G. Petrillo, T. Giani, Clinical use and molecular action of corticosteroids in the pediatric age, Int J Mol Sci, 20(2019):444.
[Crossref] [Google Scholar] [PubMed]
- M. Nosadini, J. Gadian, M. Lim, S. Sartori, T. Thomas, et al. Mycophenolate mofetil in paediatric autoimmune or immune-mediated diseases of the central nervous system: Clinical experience and recommendations, Dev Med Child Neurol, 61(2019):458-468.
[Crossref] [Google Scholar] [PubMed]
- B.D. Marjanovic, L.M. Stojanov, D.S. Zdravkovic, R.M. Kravljanac, M.S. Djordjevic, Rasmussen syndrome and long-term response to thalidomide, Ped Neurol, 29(2003):151-156.
[Crossref] [Google Scholar] [PubMed]
- A. Kumar, H. Krishnani, A. Pande, Rasmussen’s encephalitis: A literary review, Cureus, 15(2023):e47698.
[Crossref] [Google Scholar] [PubMed]
- D. Cutuli, P. Sampedro-Piquero, BDNF and its role in the alcohol abuse initiated during early adolescence: Evidence from preclinical and clinical studies, Curr Neuropharmacol, 20(2022):2202–2220.
[Crossref] [Google Scholar] [PubMed]
- F.J. Barreda Tomas, P. Turko, H. Heilmann, T. Trimbuch, Y. Yanagawa, et al. BDNF expression in cortical GABAergic interneurons, Int J Mol Sci, 21(2020):1567.
[Crossref] [Google Scholar] [PubMed]
- M. Miranda, J.F. Morici, M.B. Zanoni, P. Bekinschtein, Brain-derived neurotrophic factor: A key molecule for memory in the healthy and the pathological brain, Front Cell Neurosci, 13(2019):363.
[Crossref] [Google Scholar] [PubMed]
- G.L. Ming, H. Song, Adult neurogenesis in the mammalian brain: Significant answers and significant questions, Neuron, 70(2011):687–702.
[Crossref] [Google Scholar] [PubMed]
- L.F.I. Valdes, F.S. Humberto, Novel hypotheses on the role of oligodendrocytes in neurocysticercosis: A comprehensive research, Clin Schizophr Relat Psychoses, 17(2023).
[Crossref]
- M.N. Garcia, L.F.I. Valdés, F.S. Humberto, Our hypotheses about the role of cuproferropanoptosis in neurocysticercosis and a comprehensive review, J Drug Alcohol Res, 12(2023):9.
[Crossref]
- L.F.I. Valdes, F.S. Humberto, FerroPANoptosis in neurocysticercosis: Implications for novel therapeutic drug development-a comprehensive review, J Drug Alcohol Res, 13(2024):10.
[Crossref]
- L.F.I. Valdes, H. F. Sibat, Comorbidity of alcohol use disorders and neurocysticercosis aggravated by dysbiosis, J Drug Alcohol Res, 13(2024).
[Crossref]
- Y. Robitaille, Chronic encephalitis and epilepsy: Rasmussen syndrome. Butterworth-Heinemann. Boston, MA, USA. 1991. 79–110.
- A. L. Fornari Caprara, J. P. Rissardo, E.P. Nagele, Rasmussen encephalitis: Clinical features, pathophysiology and management strategies-A comprehensive literature review, Medicina (Kaunas), 60(2024):1858.
[Crossref] [Google Scholar] [PubMed]
- P. Maravi, S.S. Kushwaha, R. Gangwal, D. Mishra, N. Mishra, Psychiatric presentation in Rasmussen’s encephalitis, Ind Psychiatry J, 33(2024):401-405.
[Crossref] [Google Scholar] [PubMed]
Copyright: © 2025 Lourdes de Fatima Ibanez Valdes, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.